Introduction
Coronary artery disease (CAD) is the leading cause of death worldwide [1]. The formation of atherosclerotic plaques, a process known as atherogenesis, is the primary factor in the development of CAD [2]. Recent years have seen a growing interest and active research into the sex differences observed in various health conditions, including CAD. These differences are striking and have become a focal point of scientific inquiry, highlighting the need for disease prevention and treatment strategies that are tailored to sex-specific characteristics [3]. To fully understand CAD, it is essential to examine the influence of sex on its pathogenesis, especially regarding atherogenesis. Studies have identified hormonal, genetic, and lifestyle factors as contributors to the distinct patterns of disease progression observed between men and women (Fig. 1).
This review was conducted to examine the intricacies of sex differences in coronary atherogenesis, providing a foundation for more effective and personalized strategies in managing and preventing CAD. By highlighting the specific needs and risks associated with each sex, healthcare providers can better address the unique challenges presented by CAD, ultimately leading to improved outcomes and a reduction in disease incidence [4].
Ethics statement
It is a literature database-based review; therefore, neither approval by the institutional review board nor obtainment of informed consent was required.
Sex differences in coronary artery disease burden
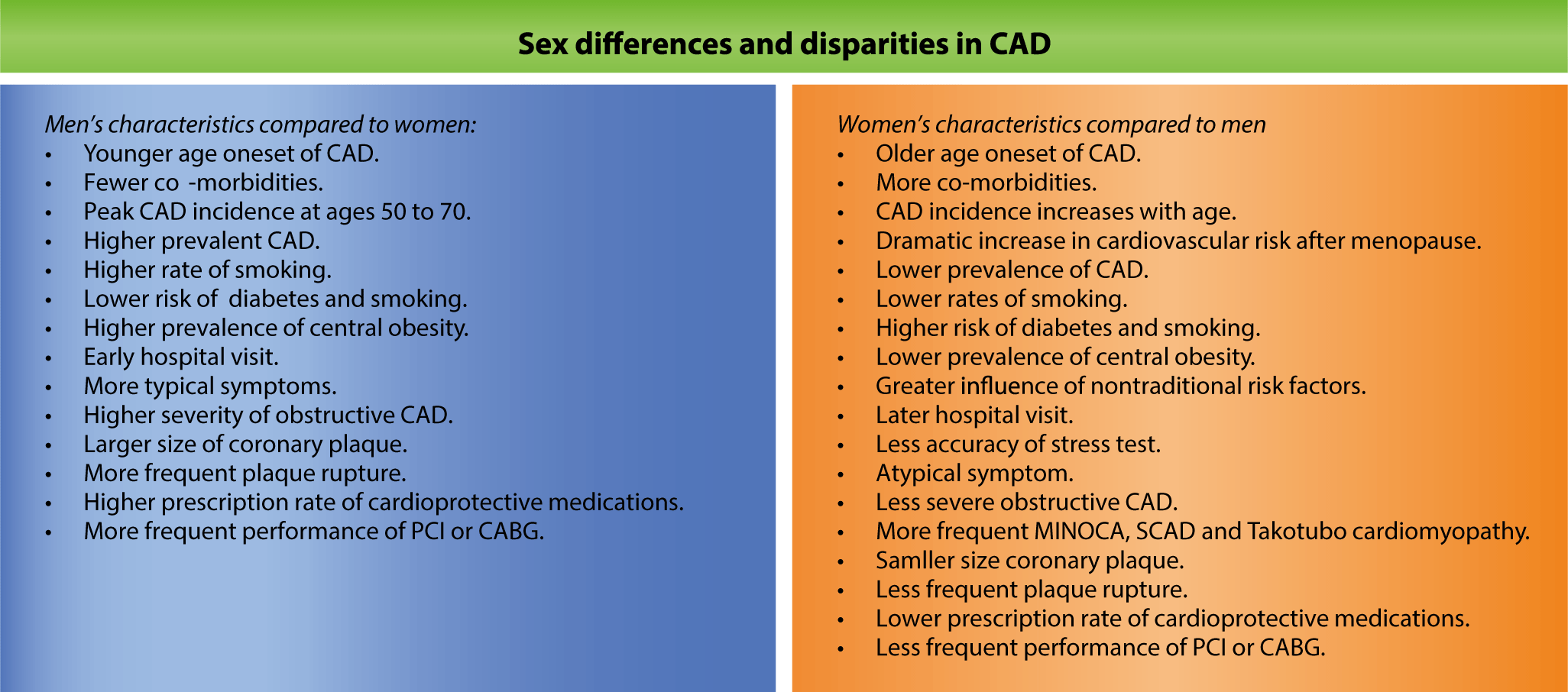
Traditionally, men have exhibited a higher prevalence and incidence of CAD compared to women. Data from the United States indicate that among all adults aged 20 years and older, the prevalence of cardiovascular disease (CVD)—which includes CAD, stroke, and heart failure—was 10.9% for men and 9.2% for women between 2017 and 2020 [1]. The age-adjusted CAD prevalence in the United States in 2018 was 7.4% for men and 4.1% for women, according to the Centers for Disease Control and Prevention. In a study of 141,459 Chinese individuals, only 31.4% of those who underwent coronary angiography (CAG) for suspected CAD were women, and among those who received percutaneous coronary intervention (PCI), just 22.2% were women [5]. A Korean study that analyzed 633,907 patients hospitalized for acute myocardial infarction (AMI) between 2002 and 2018 according to the Korean National Health Insurance Claims Database found that women accounted for 40% of the cases [6]. Several studies examining CAG findings have reported that obstructive lesions are more common and more severe in men than in women [7]. While women typically experience their first cardiovascular events later in life, the sex difference in CAD prevalence and incidence diminishes with age [1]. A study of American adults from 2008 to 2017 found that the average age of CAD onset was 57.4 years for men and 59.3 years for women [8]. Similarly, analyses of Korean patients who underwent CAG for suspected CAD or PCI showed that the average age of female patients was older than that of male patients [7,9]. According to 2021 data from the Korean Statistical Information Service, men represented the majority of overall AMI cases. However, the sex gap narrowed with age, and among individuals over 80 years old, the number of AMI cases in women was greater than that in men. In American cohort analysis data, the incidence of CAD, which was significantly higher in younger men, narrowed or even became similar to that in women aged 75 or older [10].
Role of estrogen in coronary atherogenesis
The risk of developing CAD is high for men in their 40s to 70s. In contrast, for women, the risk increases gradually with age and rises steeply from their 50s and 60s [11]. Based on analysis of the Framingham cohort, in women, 40.3% of the impact of age on CVD was found to stem from associated risk factors, greatly exceeding the 11.9% observed in men [12]. According to data from Korea’s 2013 National Health Insurance Service, men represented the majority of patients with CVD until their 50s. However, this trend reversed in the 60–69-year range, and among those in their 80s or older, 72.2% of cases were found in women and 27.8% in men.
The increased risk of CAD in older women is closely related to the decrease in systemic estrogen levels. Research suggests that the number of AMIs is negatively associated with lifetime exposure to endogenous estrogen [13], while the later onset of menopause is linked to increased life expectancy [14]. Although the exact mechanisms are not yet fully understood, estrogen exerts several beneficial effects on the cardiovascular system. Notably, this hormone promotes the relaxation of blood vessel walls, leading to vasodilation [15]. This dilation helps to lower blood pressure and improve blood flow, thereby reducing the cardiac workload. Additionally, estrogen has anti-inflammatory properties that can reduce inflammation in the arteries and decrease the risk of atherosclerosis [16]. Its antioxidant characteristics enable it to neutralize harmful free radicals, thus reducing oxidative stress and preventing damage to the walls of blood vessels and other cardiovascular tissues [17]. Furthermore, estrogen influences the distribution of body fat. After menopause, declining estrogen levels can lead to an increased risk of abdominal obesity, which in turn promotes insulin resistance and elevates cardiovascular risk [18]. Estrogen also improves cholesterol profile by increasing HDL cholesterol levels and decreasing LDL levels [19]. This action helps minimize the buildup of plaque in the arteries [20]. Additionally, estrogen supports the health and function of the endothelium, the inner lining of blood vessels, which is crucial for regulating blood vessel tone, preventing clot formation, and maintaining vascular health [21]. Overall, estrogen exerts cardioprotective effects by mitigating the risk of atherosclerosis, hypertension, and thrombosis, thereby reducing the likelihood of CVDs such as heart attack and stroke [22,23].
Sex differences in risk factors for coronary atherogenesis
As previously mentioned, age is a primary determinant of coronary atherogenesis. This factor exerts a more pronounced influence in women than in men, particularly among older individuals [12].
In individuals under 65 years old, hypertension is more prevalent in men, whereas among those over 65, the prevalence is higher in women. Women experience a marked increase in systolic blood pressure after menopause, which can be attributed to factors such as loss of estrogen, atherosclerosis, increased salt sensitivity, decreased nitric oxide levels, and a rise in angiotensin II receptors [24]. This leads to a higher prevalence of isolated systolic hypertension in women, which is a substantial risk factor for cardiovascular complications [24]. Overall, the influence of hypertension on the development of CAD and stroke appears similar between sexes [25].
Studies indicate that diabetes mellitus presents a greater cardiovascular risk for women than for men. Research involving both individuals without diabetes and those with type 2 diabetes revealed a more pronounced increase in the risk of coronary heart disease among women compared to men over a 13-year period [26]. Furthermore, a meta-analysis revealed that relative to men with type 2 diabetes, women with the condition face a 46% higher risk of mortality from coronary heart disease [27]. Another study reported that diabetes doubles the risk of occlusive vascular mortality in men and triples it in women [28]. Consequently, intensified management of cardiovascular risk is crucial for women with diabetes.
Women generally display higher levels of HDL cholesterol, whereas men are more likely to have elevated levels of LDL cholesterol. However, after menopause, women frequently see a rise in LDL cholesterol and a reduction in HDL cholesterol, increasing their risk of CVD [29]. Although sex differences in the effects of dyslipidemia on CVD are anticipated, the available data on this subject are scarce.
Like diabetes, smoking has a greater impact on the incidence of CAD in women than men. A prospective cohort study found that the risk of AMI was 1.43-fold higher in men who smoked, whereas in women, this risk was elevated by 2.24-fold [30]. Another study indicated that female smokers experienced their first AMI earlier than male smokers [31]. To mitigate the risk of CVD, increased attention should be directed toward women who smoke, and smoking cessation education programs should be implemented.
The impact of obesity on the risk of developing CAD is slightly higher in men than in women [32]. This may be attributed to the higher prevalence of abdominal obesity in men, which further elevates the risk of CVD [33]. However, following menopause, the prevalence of abdominal obesity increases in women due to estrogen depletion, with an associated increase in cardiovascular risk [18].
Nontraditional risk factors, including chronic inflammation, psychological stress, socioeconomic factors, and reproductive history, are known to influence sex differences in CAD [34].
Chronic inflammation impairs endothelial cell function, amplifies oxidative stress, and promotes vascular damage, which can lead to atherosclerosis [35]. Specifically, rheumatic diseases and autoimmune diseases, which are more prevalent in women, can trigger CAD through chronic inflammation [36].
Chronic stress elevates the secretion of stress hormones such as cortisol and adrenaline, which in turn increases blood pressure, heart rate, and inflammation. These physiological changes contribute to vascular damage and thrombus formation [37]. Although stress can induce CAD in both men and women, its effects are more pronounced in women [38].
Women tend to have lower levels of education and income compared to men, and these socioeconomic factors can contribute to the incidence of CAD. Lower socioeconomic status in women can result in restricted access to healthcare resources and preventive care for cardiovascular health [39].
Pregnancy-related factors, including gestational hypertension, preeclampsia, gestational diabetes, miscarriage, stillbirth, and low birth weight, are also associated with an increased risk of CAD [25,40]. Consequently, women with these medical histories require more proactive management that extends beyond childbirth [41].
Overall, these nontraditional risk factors are frequently overlooked and are not sufficiently addressed in patient care relative to traditional factors. To improve cardiovascular prognoses among women, efforts must be made to ensure that these nontraditional risk factors are acknowledged and proactively managed in the prevention and treatment of CAD.
Sex differences in coronary plaque
Atherosclerosis, the primary pathology underlying CAD, is an inflammatory process driven by lipids that initiates the development of plaques within arterial walls [35]. Endothelial dysfunction permits the infiltration of LDL particles into the intima, which triggers an inflammatory cascade. Adhesion molecules and cytokines facilitate the recruitment of inflammatory cells such as monocytes, neutrophils, and T cells. Monocytes differentiate into macrophages and form foam cells, which contribute to plaque formation. Vascular smooth muscle cells migrate to the intima and establish a fibrous cap, which is crucial for plaque stability. Phenotypic changes in these smooth muscle cells further stabilize plaques through extracellular matrix production [35]. Endogenous sex hormones substantially influence this process [42]. In women, estrogen reduces the expression of adhesion molecules, the infiltration of neutrophils, and the secretion of pro-inflammatory cytokines, thereby slowing the progression of atherosclerosis. After menopause, the decline in estrogen levels results in the loss of these protective effects. In comparison, men are more likely to experience plaque rupture, leading to thrombus formation due to the rupture of the fibrous cap and the exposure of thrombotic components. Plaques with thin fibrous caps and large lipid cores are particularly vulnerable to rupture. Testosterone appears to increase inflammatory cell infiltration and cytokine secretion, promoting the development of atherosclerotic lesions [42]. Autopsy data from patients after sudden coronary death indicate that men are more susceptible to the formation of blood clots and have a higher incidence of ruptures. In contrast, women are less likely to develop thrombi, and when they do, those thrombi are more likely to be associated with erosions [43]. Another study that analyzed the culprit plaques in patients with myocardial infarction found that sex exerted a greater influence on plaque characteristics than any other clinical feature [44].
Sex differences in clinical characteristics of patients with coronary artery disease
Women with CAD typically present at an older age and with a greater number of comorbidities than men. Notably, women often experience atypical angina symptoms, which can complicate the diagnostic process [45]. Functional ischemic assessments and cardiac enzyme tests are conducted less frequently in women, and these tests tend to be less accurate in detecting CAD than in men. Furthermore, conditions such as myocardial infarction with non-obstructive coronary arteries, spontaneous coronary artery dissection, and Takotsubo cardiomyopathy are more prevalent among women [46]. In addition, women who undergo PCI face a higher risk of bleeding complications than men [9].
Sex disparities in coronary artery disease
In comparison to men, women frequently delay seeking medical attention, often present with atypical symptoms, and experience lower diagnostic accuracy. These factors lead to comparatively late or missed diagnoses and subsequent delays in initiating treatment [47]. Consequently, women are less likely to undergo invasive procedures such as PCI or coronary artery bypass graft surgery compared to men [48]. Furthermore, a sex-based disparity is evident in the prescription rates of cardioprotective drugs. Relative to men, women are less frequently prescribed essential medications such as antiplatelets, renin-angiotensin system blockers, and statins, which are crucial for managing CVD [49]. Moreover, women are significantly underrepresented in clinical research related to CAD [50]. These sex disparities ultimately lead to poorer cardiovascular prognoses among women.
Future directions
As mentioned above, sex differences are evident in the pathophysiology, risk factors, clinical manifestations, and treatment responses of CAD, as well as in diagnostic and therapeutic approaches. However, most clinical studies have not collected data on female-specific risk factors, such as pregnancy history, age at menopause, and polycystic ovary syndrome, and have not included this information in their analyses. Historically, clinical research has been male-dominated, with women frequently excluded due to factors related to fertility, breastfeeding, or menopause [51]. Awareness must be raised of these sex differences and disparities, not only among healthcare professionals but also within the general population. A survey of middle-aged and elderly women in Korea revealed a very low level of awareness regarding heart disease in women [52]. The “Go Red for Women” campaign, initiated by the American Heart Association in 2004, has raised awareness of CVD in women and effectively reduced cardiovascular mortality within this population, highlighting the impact of such awareness campaigns [53,54]. Furthermore, knowledge of sex-based differences must be integrated into clinical guidelines [34]. To conduct sex-specific analyses with sufficient statistical power, the anticipated number of patients must be at least doubled. However, creating separate guidelines for each sex presents several challenges and can be both complex and impractical. A more feasible approach in clinical practice is to address sex differences within a unified set of guidelines. In many developed Western countries, CAD recommendations consistently underscore the importance of considering sex differences. For instance, guidelines from these countries often highlight the need for cardiovascular care for women after childbirth [55]. Clinicians and researchers should be cognizant of these differences and routinely assess them in their clinical work and research endeavors. By establishing guidelines grounded in robust evidence, and by increasing the inclusion of women in randomized trials and conducting comprehensive analyses of sex differences, high-quality evidence can be progressively amassed and reflected in future recommendations [56].
Conclusion
Stark differences exist between men and women in terms of CAD burden, risk factors, plaque characteristics, and clinical features. These distinctions highlight the need for tailored diagnostic and therapeutic approaches for CAD in both sexes. It is also essential to recognize the longer diagnostic timelines, delays in procedures/surgery, and lower rates of medication prescriptions in women. Addressing these issues requires an increased awareness of sex differences and discrimination, as well as greater inclusion of women in clinical research to gather more complete data on women’s cardiovascular health.
