Introduction
Gastric cancer (GC) represents the fifth most common malignancy worldwide and exhibits the highest incidence rates in Eastern Asia, including South Korea [1,2]. Traditional risk factors for GC encompass Helicobacter pylori infection, dietary patterns, and exposure to risk factors such as alcohol consumption and smoking [3–5]. Furthermore, the influence of obesity on the development of non-communicable diseases, including GC, has become more pronounced [6], paralleling the global rise in obesity rates [7].
Despite considerable interest and research, the relationship between obesity and GC remains less clear than for other cancers, such as colon cancer [8,9]. This ambiguity is partly because the pathogenesis of GC varies by anatomical location. Cardia and non-cardia GCs each have unique pathological and etiological features [10]. In cardia cancers, the risk of developing GC due to obesity is higher, and the association with obesity is more pronounced. In contrast, non-cardia cancers do not exhibit a significant link with obesity [11]. However, a recent study from Korea suggested that underweight was associated with an increased risk of developing GC [12], with a U-shaped pattern of risk increase. Therefore, the relationship between underweight or overweight and the risk of developing GC necessitates further investigation. GC is also recognized as a male-dominant disease [13], with this sex difference typically attributed to variations in exposure to risk factors and the influence of sex hormones [14,15]. The effect of weight outside the normal range on the development of GC likely differs between sexes, given that estrogen, a key sex hormone, is associated with obesity [15].
Methods
The study protocol was approved by the institutional review board (IRB) of Seoul National University Bundang Hospital (IRB No. X-2209-780-901). The requirement for informed consent was waived by the IRB.
This retrospective cohort study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (https://www.strobe-statement.org/).
In February and March of 2024, the authors conducted a search regarding BMI and GC within the National Health Insurance Service–Health Screening Cohort (NHIS-HEALS) database, which contains records from 2002 to 2019. The selected data were subsequently analyzed by the authors.
Korea operates the National Health Insurance Service (NHIS), a single, mandatory health insurance system that covers approximately 97% of the Korean population. The NHIS administers a biennial health checkup program for adults aged 20 years and older, known as the National Health Screening (NHS). The NHS program encompasses over 70% of the total population in Korea [16]. During these NHS health checkups, various data are collected from the examinees. These include anthropometric measurements such as height, weight, and waist circumference (WC), as well as sociodemographic factors and health behaviors, including alcohol consumption and smoking status. Additionally, family and medical histories are recorded alongside laboratory test results [16,17].
For research purposes, the NHIS constructed a sampled retrospective cohort consisting of 514,866 participants. These individuals were randomly selected from participants in the NHS programs in 2002–2003, a cohort referred to as NHIS-HEALS. This cohort has undergone annual follow-up through 2019 to gather information on healthcare utilization and mortality.
Since the NHS has collected WC data since 2009, we defined 2009–2010 as the baseline period. Among the participants in the NHIS-HEALS (n=514,866), those lacking information on BMI for 2009–2010 were excluded. Additionally, we excluded participants with a history of cancer, operationally defined as those with claims data containing a major diagnosis code beginning with “C” from 2002–2008. In Korea, the NHIS offers a program that enables adults over 40 years of age to undergo GC screening every 2 years. We posited that a minimum of 2 years is necessary for BMI at a given time point to influence the development of GC. Consequently, GC cases diagnosed within 2 years following BMI measurement were excluded. Ultimately, 341,999 participants, including 4,277 GC cases, were selected for the study (Fig. 1).
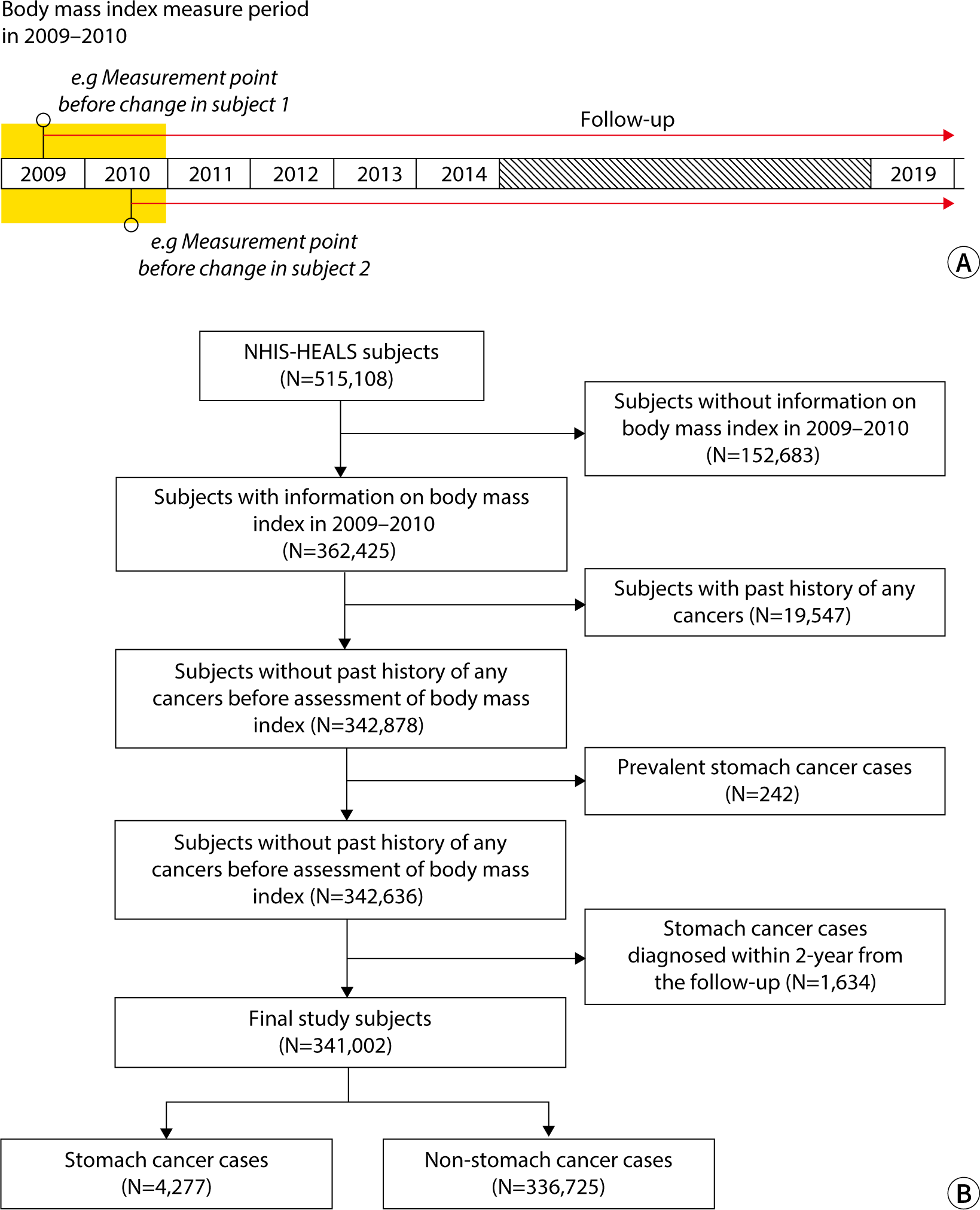
Study participants were categorized into four groups based on their BMI, following the classifications used in a prior study [18]: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5–22.9 kg/m2), overweight (BMI 23.0–24.9 kg/m2), obesity (BMI 25.0–29.9 kg/m2), and severe obesity (BMI ≥30.0 kg/m2). Additionally, WC reference values of 90 cm for male and 85 cm for female participants were adopted in line with the Korean diagnostic criteria for metabolic syndrome [18].
We operationally defined GC cases as participants who had medical claims with the corresponding diagnosis code for GC, specifically the International Classification of Diseases-10 code C16, along with a history of hospital admission. The follow-up period was defined as the time from the index date (the date of BMI measurement) to the date of GC diagnosis, death, or the end of the follow-up period (December 31, 2019), whichever occurred first.
The outcome variables included demographic findings, such as a diagnosis of GC, along with age, WC, and BMI.
Since participants were selected from the cohort database according to the inclusion criteria, selection bias was not a concern.
To compare differences in baseline characteristics across BMI levels, we conducted univariable tests, utilizing the chi-square test for categorical variables and analysis of variance for continuous variables. The characteristics considered included sociodemographic factors (such as age, sex, and income), health behaviors (including smoking status and physical activity), fasting glucose level, total cholesterol, blood pressure at the time of BMI measurement, and comorbidities such as diabetes mellitus, hypertension, and dyslipidemia recorded before the most recent BMI measurement. We assessed GC risk in relation to BMI by using the overweight group (BMI of 23.0–24.9 kg/m2) as the reference category in Cox proportional hazard regression analysis.
To account for the influence of baseline characteristics on the development of GC, we constructed a Cox proportional hazards regression model. This model included age, sex, alcohol consumption, smoking status, and history of diabetes mellitus, hypertension, and dyslipidemia as exploratory variables.
Although some baseline characteristics (namely, smoking status and alcohol consumption) had missing values, the proportions of missing data were relatively low: 1.9% for smoking status and 3.1% for alcohol consumption. To address the issue of missing values, we conducted data imputation using the PROC MI procedure within SAS (SAS Institute, Cary, NC, USA).
All data management and statistical analyses were performed using SAS version 9.4. The protocol of this study was approved by the IRB of Seoul National University Bundang Hospital (IRB No. X-2209-780-901).
Results
The baseline characteristics of the participants are summarized in Table 1. Most participants fell into the normal weight, overweight, and obesity categories, with only a small percentage classified as underweight (2.1%) or having severe obesity (2.8%). Among BMI categories, the severe obesity group contained the highest proportions of women, non-smokers, and non-drinkers. Furthermore, the prevalence rates of diabetes, hypertension, and dyslipidemia increased with rising BMI levels.
Although we hypothesized that both underweight and obesity were associated with an increased risk of GC development, only those with obesity (BMI ≥25.0 kg/m2) exhibited an elevated GC risk compared to participants with a BMI of 23.0–24.9 kg/m2 (BMI 25.0–29.9 kg/m2: hazard ratio [HR], 1.11; 95% CI, 1.03–1.20; BMI ≥30.0 kg/m2: HR, 1.22; 95% CI, 1.01–1.47). However, an elevated GC risk was not observed in the underweight population in this study. Although the highest increase in GC risk was found in those with severe obesity, this association was only noted in the male population (HR, 1.36; 95% CI, 1.12–1.74).
In a sensitivity analysis considering various latency periods, we assessed the association between BMI and GC risk by repeatedly excluding GC cases diagnosed within 2, 3, and 4 years of follow-up (Table 2). The analysis showed that the increase in GC risk was consistently higher with greater severity of obesity, regardless of the latency period considered. Furthermore, a significantly elevated GC risk in the severe obesity group was observed exclusively in the male population. Consequently, we applied a 2-year latency period to subsequent analyses.
Adjusted for age, sex, smoking status, alcohol consumption frequency per week, history of diabetes mellitus, hypertension, and dyslipidemia with consideration of a 2-year latency period.
A stratification analysis by age was conducted using a threshold of 50 years (Table 3). Notably, the NHIS-HEALS dataset was characterized by a disparity in participant person-years, with approximately 49,000 for individuals under 50 years and over 3 million for those aged 50 and above. This resulted in limitations regarding the statistical power for the subset of participants younger than 50 years.
Adjusted for age, sex, smoking status, alcohol consumption frequency per week, history of diabetes mellitus, hypertension, and dyslipidemia with consideration of a 2-year latency period.
In individuals under 50 years old, severe obesity (BMI ≥30.0 kg/m2) was associated with a heightened risk of GC in men (HR, 1.83; 95% CI, 0.99–3.37). However, no significant associations between BMI and GC risk were found in the other BMI categories.
In participants over 50 years of age, we observed an increased risk of GC in both men and women with obesity, which is consistent with the results of the preceding analysis. Furthermore, an elevated risk of GC was also identified in women with normal BMI (18.5–22.9 kg/m2).
Additional analysis considering WC was performed to account for abdominal obesity, as BMI only reflects the height and weight of participants (Table 4). We aimed to clarify the impact of underweight on the development of GC in those with a low WC (<90 cm for men and <85 cm for women), as well as the impact of obesity on GC development in the group with a high WC (≥90 cm for men and ≥85 cm for women). Even among those with a small WC, we observed no significant increase in GC risk among underweight men (men: HR, 1.10; 95% CI, 0.87–1.40; women: HR, 1.01; 95% CI, 0.65–1.57). However, we did find that the risk of GC increased in the male population with severe obesity and a WC of ≥90 cm. Furthermore, the magnitude of increased GC risk in men with severe obesity and a high WC (HR, 1.41; 95% CI, 1.07–1.85) was greater than that observed when WC was not considered (HR, 1.36; 95% CI, 1.12–1.74). These results suggest that obesity, particularly abdominal obesity as opposed to simple weight gain, plays a role in elevating the risk of GC.
Adjusted for age, sex, smoking status, alcohol consumption frequency per week, history of diabetes mellitus, hypertension, and dyslipidemia with consideration of a 2-year latency period.
Discussion
In this study, we aimed to analyze the influence of BMI on GC risk according to sex, based on a large-scale retrospective cohort analysis. The results indicated that GC risk was higher in the obesity and severe obesity groups. Furthermore, the increase in GC risk was particularly pronounced in women with obesity and men with severe obesity. Normal BMI was also associated with increased GC risk in women aged 50 years and older. When considering BMI and WC together, the risk of GC was elevated in men with severe obesity and high WC.
Previous studies have identified a heightened risk of GC associated with overweight or obesity, particularly concerning cardia cancer [11,19–25]. These studies suggest that both overweight and obesity are linked to an increased risk of cardia cancer. Consequently, the World Cancer Research Fund and the International Agency for Research on Cancer have recognized overweight and obesity as risk factors for cardia GC [26,27]. However, no significant difference in the risk of non-cardia GC has been observed between individuals with normal weight and those with obesity [11]. One proposed mechanism for the link between obesity and cardia cancer involves the development of gastroesophageal reflux disease (GERD) due to obesity [28,29]. Specifically, the rise in intra-abdominal pressure caused by abdominal obesity may lead to the reflux of gastric acid and the gastric contents, resulting in a higher incidence of GERD among individuals with obesity [30]. Consequently, obesity could increase the risk of cardia GC through a cascade of events, extending to GERD to Barrett esophagus and ultimately to gastroesophageal junction adenocarcinoma, given that Barrett esophagus is recognized as a precursor to esophagogastric junction adenocarcinoma [31,32]. Additionally, other mechanisms such as hyperinsulinemia and an increase in insulin-like growth factors, as well as elevated levels of adipokines (leptin and adiponectin), tumor necrosis factor-alpha, and interleukin 6 secreted from adipose tissues, have been proposed as potential contributors to the increased risk of GC under obese and diabetic conditions [33,34].
Our data revealed a sex difference in the increased risk of GC, with the difference being more pronounced in men with obesity [35]. This may be attributed to the distinct patterns of obesity between the sexes. Typically, men exhibit a central distribution of adipose tissue, while women tend to have a peripheral distribution, particularly in the limbs and hips [36]. The greater visceral adiposity found in central obesity is linked to adverse metabolic outcomes, including increased postprandial insulin, free fatty acids, and triglyceride levels [36]. Given that factors secreted from adipose tissues have been proposed among potential mechanisms for elevating GC risk, this could provide an explanation. Another factor to consider is sex hormones. The aforementioned sex-based obesity patterns are largely due to variations in sex hormones and their receptors. For instance, estrogen is known to exert a protective effect against GC, particularly the intestinal type [37]. This protective role of estrogen has been repeatedly proposed to account for the disparity in GC risk between male and female individuals [14,38,39]. Estrogen is synthesized not only in the gonads, but also in adipose tissue. Thus, its levels may become particularly high in obese women, which could further explain the sex difference in GC risk within the obese population. However, few studies have concurrently considered BMI, sex, and the anatomical location of GC, which complicates the ability to draw definitive conclusions. Therefore, additional research in this area is warranted.
Another notable result of this study is the observed association between normal weight and an increased risk of GC in women aged 50 years and older. Recent reports have indicated a rise in GC risk among both underweight and overweight individuals, forming a so-called “U-shaped pattern” [12,40]. While the link between overweight, obesity, and GC risk has been described previously, it appears that being underweight or of normal weight also increases GC risk compared to those who are overweight. To explain this phenomenon, the authors propose two potential mechanisms. The first suggests that precursor lesions of GC, such as atrophic gastritis or metaplasia, could lead to malabsorption and consequently underweight [41]. This theory is supported by the fact that the underweight group in our study was older than the other groups. The second mechanism posits that cigarette smoking could link underweight to an increased risk of GC [12]. Additionally, it is worth considering that being underweight might cause gonadal dysfunction or a decrease in estrogen levels, which could negate the protective effects of estrogen. To date, the causal relationship or sequence of events linking underweight or normal weight with GC risk remains unclear. Moreover, one previous study found an association between underweight and non-cardia cancer [12], while another study reported a link between underweight and cardia cancer [40], indicating some inconsistencies in the findings. In conclusion, when investigating the correlation between obesity and GC risk, it is essential to consider not only sex but also age and the anatomical location of the cancer.
This study has several limitations. First, since WC data have been collected since 2009 from the NHIS-HEALS dataset, we analyzed data with a relatively short follow-up period compared to other observational studies. Second, we examined all GC cases without differentiating between anatomical locations—namely, cardia or non-cardia sites. To address these issues, we plan to conduct long-term follow-up studies using BMI for comparison. Third, our age-specific analysis was constrained by the small number of participants and cancer cases under the age of 50 years, which reflects the characteristics of the NHIS-HEALS dataset. Given the rising concern over GC in younger populations, future studies should include datasets with a larger representation of young adults. Lastly, we were unable to account for other GC risk factors, such as H. pylori infection status and dietary habits, due to the inherent limitations of observational studies. Despite these constraints, we were able to identify the risk of developing GC associated with weight gain, while noting some sex differences.
This study demonstrated a positive association between excess body weight and the risk of GC in Koreans, indicating that the risk of GC was elevated in individuals with obesity and severe obesity. Additionally, sex-specific differences were observed in the impact of obesity on GC development, with men who were severely obese and had a high WC facing a particularly increased risk.
