Introduction
Antibiotics were originally defined as substances produced by microorganisms that inhibit the growth or proliferation of other microorganisms. This definition has since been expanded to include artificially synthesized compounds. Since the discovery of penicillin, the development and use of various antibiotics have markedly reduced mortality from infectious diseases, positioning antibiotics as one of the most transformative interventions in modern society. This progress has led to bold predictions that humanity might one day conquer bacterial infections. Despite these optimistic projections, bacteria have survived by employing various mechanisms that nullify the effects of antibiotics. The resulting resistant bacteria have proliferated, leading to the emergence of multidrug-resistant organisms (MDROs) that are unresponsive to conventional treatments. The development of antibiotic resistance is far outpacing the introduction of new antibiotics [1], and multiple studies have highlighted the harmful impacts of antibiotic resistance on socioeconomic and public health indicators, including healthcare costs, length of hospitalization, and mortality [2,3]. In response, at the 68th World Health Assembly in 2015, the World Health Organization (WHO) declared antibiotic resistance a critical threat to human life, calling for international action plans to address the issue across borders. While antibiotic resistance is recognized as a key global challenge, Korea exhibits higher antibiotic prescription rates and antimicrobial resistance than many other countries [4]. The awareness of antibiotic resistance in Korea has gradually improved, with interventions reducing unnecessary antibiotic prescriptions; however, resistance rates remain comparatively high, and some multidrug-resistant bacteria are even on the rise [5]. In particular, the reported incidence of carbapenem-resistant Enterobacterales (CRE), which is increasing worldwide, is also gradually climbing in Korea. With limited antibiotic options available for treating CRE, its increased incidence poses a management challenge, underscoring the importance of prevention and preemptive strategies.
Ethics statement
As this study is a literature review, it does not require institutional review board approval or individual consent.
Status of multidrug-resistant organisms in Korea
In 2009, Korea enacted the Infectious Disease Control and Prevention Act, which classified six multidrug-resistant bacterial infections as designated communicable diseases. These included methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), vancomycin-resistant enterococci (VRE), multidrug-resistant Pseudomonas aeruginosa (MRPA), multidrug-resistant Acinetobacter baumannii (MRAB), and CRE. The Korean government established a sentinel surveillance system for healthcare-associated infectious diseases, including these six types of MDROs. In 2017, CRE and VRSA were reclassified as Group 3 infectious diseases, necessitating continuous surveillance and mandatory reporting. In 2020, the Infectious Disease Prevention Act was amended, changing the legal classification system from groups to classes. Consequently, VRSA and CRE were reclassified as Class 2 infectious diseases, and a mandatory surveillance system for these strains has been maintained to date. MRSA, MRPA, and MRAB are designated as Class 4 infectious diseases and are monitored through sentinel surveillance.
In May 2016, aligning with the international effort to combat antibiotic resistance, Korea established the Korea Global Antimicrobial Resistance Surveillance System (Kor-GLASS). This system was modeled after the Global Antimicrobial Resistance and Use Surveillance System (GLASS) proposed by the WHO to assess the national status of antimicrobial-resistant bacteria [6]. Kor-GLASS has been supplemented and adapted to reflect domestic conditions, building upon the foundation provided by GLASS [6]. Since its inception in 2016, Kor-GLASS has collected data on 12 pathogens, including Escherichia coli, Klebsiella pneumoniae, S. aureus, Enterococcus spp., Acinetobacter spp., and P. aeruginosa. These bacteria are gathered from nine general hospitals across the nation, with the system confirming antibiotic susceptibility test results and computing resistance rates.
Based on the annual antibiotic resistance rates among the six key strains from 2016 to 2022, as reported in the Kor-GLASS data (Fig. 1), the resistance rate of S. aureus to methicillin has been continuously declining since 2016. This trend aligns with observations in other developed countries [7,8]. To date, no VRSA strains have been identified in Korea. Suspected strains have been referred to provincial public health and environment research institutes, as well as the Korea Disease Control and Prevention Agency, for confirmation. However, all have been identified as vancomycin-intermediate S. aureus strains [9]. Regarding VRE, carbapenem-resistant P. aeruginosa, and carbapenem-resistant K. pneumoniae (CRKP), the incidence of infections caused by resistant bacteria has been rising since the initiation of sentinel surveillance. This increase coincides with the escalated use of broad-spectrum antibiotics and mirrors global trends. During the coronavirus disease 2019 pandemic, Korea, like the United States, experienced an uptick in multidrug-resistant bacterial infections. Several factors may have contributed to this pattern, including increased antibiotic prescriptions for patients with respiratory symptoms, the saturation of isolation facilities in medical institutions, staff shortages, and challenges in adhering to infection control guidelines due to work overload [10].
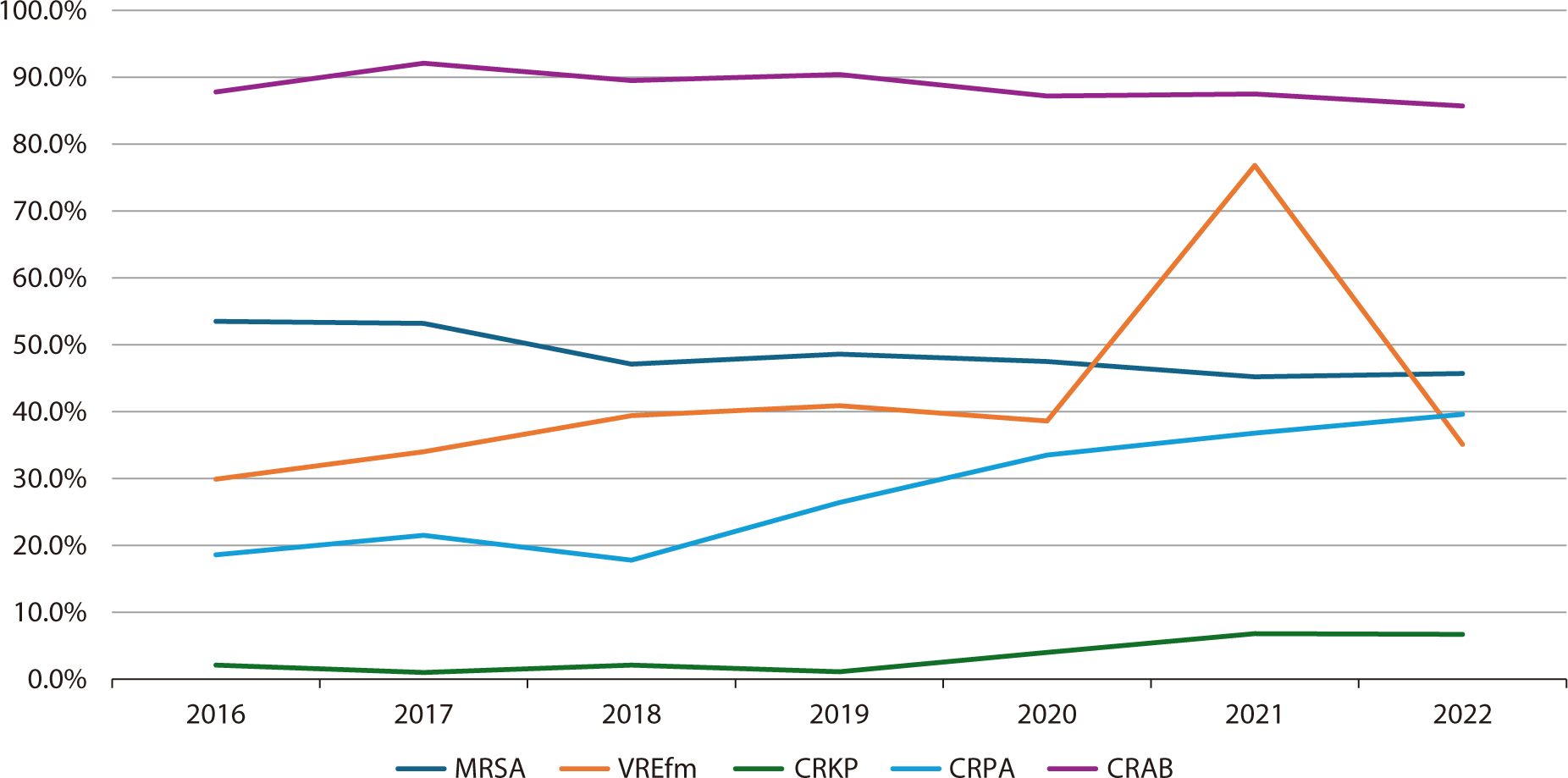
Status of carbapenem-resistant Enterobacterales in Korea
Among the six MDROs, CRE is particularly noteworthy. In 2017, CRE was classified as a Group 3 infectious disease. Following the revision of the Infectious Disease Prevention Act in January 2020, CRE was reclassified as a Class 2 infectious disease. This reclassification mandates that medical personnel report cases within 24 hours in the event of an outbreak or epidemic, as part of a mandatory surveillance system. The reporting rate of CRKP, which has been collected and monitored by Kor-GLASS since 2016, has been continuously increasing. Similarly, the number of reported CRE infections from medical institutions has been rising annually, with an accelerating growth rate (Fig. 2) [11].
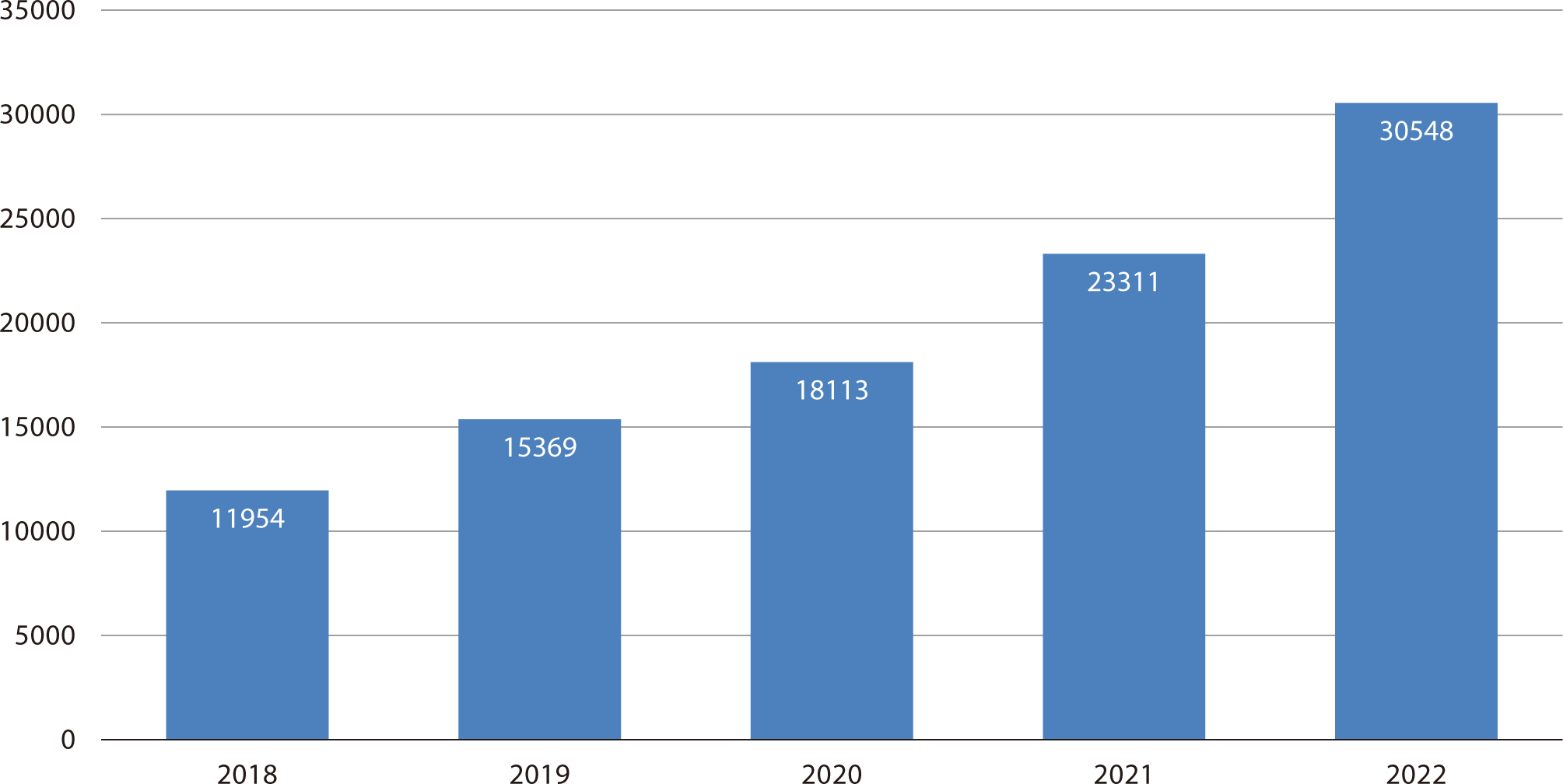
In 2022, K. pneumoniae was identified as the predominant CRE species, comprising 70.9% of isolated strains, followed by E. coli (14.0%) and Enterobacter spp. (7.0%). K. pneumoniae consistently emerged as the most prevalent species throughout the surveillance period [11]. CRE can be categorized based on the mechanism of carbapenem resistance. Carbapenemase-producing Enterobacterales (CPE) produce enzymes that degrade carbapenems, while non-CPE bacteria demonstrate resistance through other means such as efflux pumps, changes in outer membrane protein permeability, or overproduction of AmpC beta-lactamase or extended-spectrum beta-lactamases. In Korea, CPE accounted for 83.0% of all CRE cases in 2021, surpassing non-CPE pathogens, and this proportion has been rising. The carbapenemases identified to date include K. pneumoniae carbapenemase (KPC), New Delhi metallo-beta-lactamase (NDM), Verona integron-encoded metallo-beta-lactamase (VIM), imipenemase (IMP), and oxacillinase-48 (OXA-48) [12]. Since 2018, KPC has been the most common carbapenemase among domestic CPE strains, followed by NDM and OXA (Fig. 3) [13,14]. The distribution of carbapenemases varies by region and country, with the transmission of new carbapenemases being reported in developed countries [15]. Consequently, maintaining up-to-date molecular epidemiological information on CRE, assessing the domestic context, and performing continuous monitoring of its spread and outbreaks are crucial.
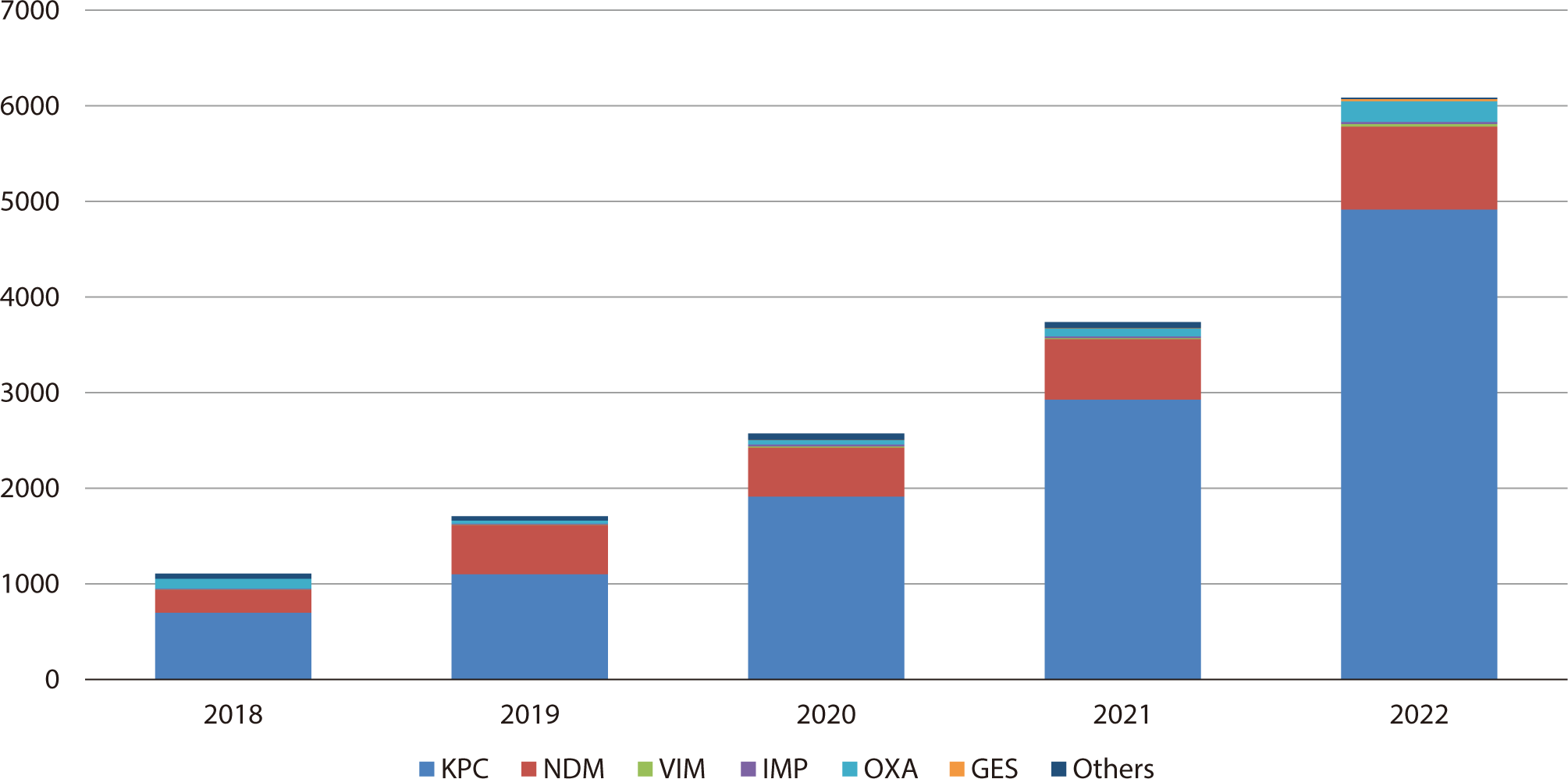
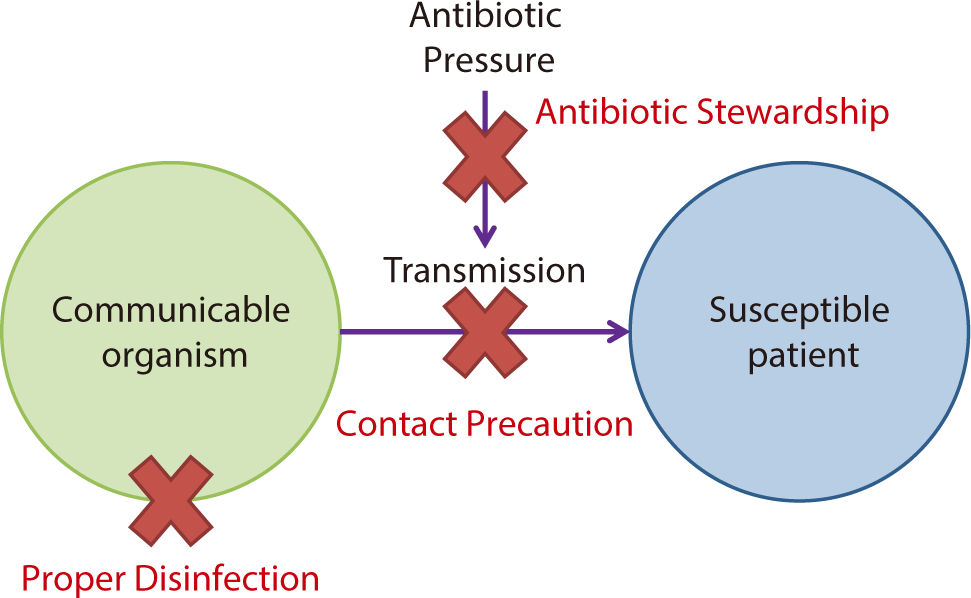
Interventions to prevent carbapenem-resistant Enterobacterales transmission
Infection control strategies to prevent the spread of CRE can be organized into three main components (Fig. 4). Considering the process that facilitates CRE transmission within hospital settings, the first component involves selective pressure from antibiotic use, which creates a favorable environment for CRE to survive and proliferate. The second aspect is the transmission from CRE-infected or colonized patients to others via the hands of healthcare workers or medical devices. The third component is the spread of CRE to the surrounding healthcare environment, where it can form clusters and foster conditions that promote further transmission. At each stage, adherence to an antimicrobial stewardship program (ASP) is crucial to combat the survival advantage of resistant bacteria. Additionally, early screening and isolation of CRE carriers, contact precautions to prevent transmission, and environmental disinfection to eradicate colonies can be effective strategies to inhibit the spread of CRE.
Since long-term exposure to broad-spectrum antibiotics is a key risk factor for CRE infection [16,17], adherence to ASPs is essential for reducing the incidence of MDRO infections [18,19], including CRE. ASPs are interventions designed to guide medical staff in selecting appropriate antibiotics and using them for the correct duration. The goal of an ASP is to improve patient safety, reduce healthcare costs and treatment failures, and limit the emergence of multidrug-resistant bacteria. Through the ASP, the selective pressure on CRE can be lessened by curbing the inappropriate use of antibiotics by healthcare providers. ASPs can be implemented through various practical strategies, such as limiting excessive antibiotic use through antibiotic approval programs and establishing clinical guidelines for standardized first-line antibiotic selection and de-escalation [20]. However, the effectiveness of an ASP is not solely dependent on the participation and adherence of individual healthcare providers who prescribe antibiotics. It also relies on the establishment of policies and cultures at both national and societal levels that enable healthcare providers to engage in and comply with the ASP [21,22]. For instance, in Korea, measures such as incorporating ASPs into healthcare accreditation criteria and evaluating fees for infection prevention and control can act as additional incentives for healthcare providers to adhere to an ASP [23]. Moreover, as the reporting rate of CRE in long-term care facilities (LTCFs) is the second highest after general hospitals [11], and CRE colonization in LTCFs is considered a global risk factor for CRE transmission, it is imperative to establish an integrated ASP that encompasses various healthcare settings, including acute care institutions, LTCFs, and primary, secondary, and tertiary medical institutions. Specific implementation strategies should be tailored to each hospital context [24].
CRE is predominantly transmitted within the hospital setting through direct or indirect contact with infected individuals or via contaminated surfaces and environments. Hand hygiene has been recognized as the most effective method for interrupting this mode of transmission for multidrug-resistant bacteria and healthcare-associated infections, as evidenced by multiple studies [25,26]. Infection control guidelines consistently advocate for the implementation of and adherence to standard and contact precautions, which include hand hygiene, to prevent and manage CRE. These guidelines also encourage the use of personal protective equipment and the allocation of individual medical devices for each patient [27]. The WHO guidelines specifically advise that patients colonized or infected with CRE should be physically separated from those who are not, preferably in single-room isolation. When this is not feasible, cohorting patients with the same resistant pathogen is recommended. Additionally, dedicated medical staff should be assigned to care for these patients to minimize the risk of CRE transmission [28]. Monitoring hand hygiene practices is crucial in preventing the spread of CRE and other multidrug-resistant bacteria. Hospitals must ensure that hand sanitizers and other necessary resources are readily available to healthcare workers to facilitate consistent hand hygiene practices [29,30].
Implementing infection control measures, such as preemptive isolation, at an early stage through CRE surveillance is widely recognized to reduce CRE infections [31]. Guidelines for the prevention of healthcare-associated infections, published by the Korean Disease Prevention and Control Agency, recommend conducting CRE surveillance for groups at high risk [27]. These groups include patients transferred from hospitals with a high prevalence of CRE, critically ill individuals, and those being considered for admission to the intensive care unit with risk factors such as invasive catheter placement or exposure to broad-spectrum antibiotics. For these high-risk groups, a screening test is performed by collecting a stool or rectal swab specimen at the time of admission. The modified Hodge test is a phenotypic assay initially developed as a test for CPE. It has been widely used due to its very high selectivity for KPC-producing CPE—the most common form in Korea—and economical nature. However, since 2018, it has been excluded from the methods recommended by the Clinical and Laboratory Standards Institute due to its subjective interpretation and low sensitivity of around 50% for NDM-producing strains. Molecular assays, such as polymerase chain reaction, are the most expensive of the CPE screening methods but have benefits including quick confirmation (within 4 to 6 hours) and high sensitivity. Culture-based test methods have lower sensitivity than molecular genetic methods and require substantial effort and time, but they are considered cost-effective [32]. CRE can rapidly spread within healthcare facilities because resistance genes such as carbapenemases can be horizontally transferred to other bacteria through plasmids or transposons, and vertical transmission by a single clone is also possible [33,34]. Therefore, rapid diagnosis and response are crucial for inhibiting CRE transmission. Furthermore, the implementation of an ASP requires considerable time to clearly impact the spread of CRE. Additionally, in the Korean context, limitations on staffing and time hinder the application of ASPs [35]. Thus, swiftly diagnosing CRE infections through screening tests and subsequently responding can provide complementary assistance in managing CRE transmission. Previous studies have similarly confirmed that rapid screening tests can reduce the incidence of CRE infections [36,37]. To obtain rapid results, methods such as culturing on chromogenic media (Chromagar KPC, Imipenem-MacConkey method, etc.) followed by confirmation of CPE genotypes using the Carba NP test or immunochromatography can be used [38]. Specifically, commercially available early diagnostic kits can simultaneously detect and differentiate five major carbapenemases— KPC, NDM, OXA-48, IMP, and VIM, which are common in Korea—within 1 to 2 hours using automated equipment [39]. However, these early diagnostic kits can only detect a limited number of enzymes. Furthermore, for clinical application, an additional systematized approach is required to utilize the test results for preemptive isolation or promptly switch to appropriate antibiotics through real-time feedback. Moreover, clearly defined criteria must be available regarding patient selection for rapid diagnostic kits, considering costs, human resources, and other factors specific to each healthcare setting.
The hospital environment can serve as a reservoir for CRE; thus, environmental disinfection is key to preventing CRE transmission. Per the WHO guidelines, CRE isolation rooms should undergo additional cleaning and disinfection, with regular assessments to ensure compliance with environmental cleaning and disinfection protocols [28]. The US Centers for Disease Control and Prevention guidance on CRE management highlights the potential for CRE colonization in sink drains in inpatient rooms. Consequently, it is critical to clean areas around sinks that are prone to aerosol generation. After patient discharge, comprehensive room disinfection and rigorous monitoring are required to confirm that all surfaces have been adequately disinfected [40]. In short, rooms that have accommodated patients with CRE infection can become a source of infection. It is imperative to disinfect all surfaces, with particular attention to sinks, drains, and faucets, which are recognized as common sites for bacterial colonization [41].
Additionally, both chlorhexidine gluconate baths and staff-focused infection control education contribute to reducing the proportion of CRE carriers, despite their absence from domestic guidelines [42]. Although directly applying recommendations to the Korean context may pose challenges, it is essential to develop CRE transmission prevention guidelines that are tailored to the domestic situation, drawing on local research and evidence.
Conclusion
The ongoing increase in MDROs that are unresponsive to various antibiotics represents a key global public health challenge. In Korea, the incidence of MDRO infections is on the rise, mirroring worldwide trends. This pattern has been identified through the implementation of antibiotic resistance surveillance systems (such as Kor-GLASS) that adhere to international standards, as well as by monitoring critical antibiotic-resistant bacteria that are classified as legal infectious diseases. Thus, continuous surveillance to accurately assess antibiotic resistance is essential for preventing the spread of MDROs. Kor-GLASS currently excludes primary and secondary hospitals, LTCFs, and certain regions, indicating a need for supplementation to establish a comprehensive surveillance system. The establishment of a national real-time alert system, coupled with data sharing between the government, acute care hospitals, and LTCFs, is anticipated to provide additional support in curbing the transmission of MDROs, as observed in other countries.
CRE exhibit resistance to various broad-spectrum antibiotics, including carbapenems, which leads to high mortality rates due to the limited treatment options available. Infections caused by CRE not only raise healthcare costs and place a burden on the healthcare system but also contribute to the spread of healthcare-associated infections through an increase in pathogen carriers. Prevention is paramount in curbing the continuous growth of CRE cases. Individual healthcare providers must adhere to ASPs and minimize unnecessary antibiotic use. Concurrently, robust social systems must be established to support healthcare providers in complying with ASP guidelines. Additionally, it is vital to prevent further transmission through preemptive isolation and surveillance testing of patients at high risk, such as individuals transferred from LTCFs or admitted to intensive care units. While early diagnostic kits can be applied for rapid diagnosis, it is advisable to weigh the advantages and disadvantages of these tests and to use them judiciously, tailored to the circumstances of each medical institution. Beyond the use of early diagnostic kits, infection control interventions—including contact isolation, hand hygiene, environmental cleaning and disinfection, and the education of healthcare workers—should be implemented in conjunction with ASP practices. Ongoing research to verify the effectiveness of these infection control strategies in Korea is essential. Based on the findings, CRE management guidelines suitable for the domestic situation should be developed to curb CRE infections.
