Introduction
Hypertension and type 2 diabetes (T2D) are well-known cardiovascular risk factors. Additionally, obesity, smoking, alcohol consumption, and decreased physical activity are established risk factors associated with hypertension or T2D [1,2]. Previous studies have highlighted that lifestyle modifications can significantly reduce mortality risks in patients with hypertension or T2D [3,4]. However, once patients are diagnosed with hypertension or diabetes, they often begin treatment with medication, which means that analyses on the effect of lifestyle modifications are very limited. Previous research has primarily focused on how one specific lifestyle change affects hypertension or T2D. Moreover, studies examining whether lifestyle modifications have a similar clinical effect on cardiovascular events or mortality in the prehypertension or prediabetes stages are somewhat scarce. For example, a weight loss of approximately 10 kg may reduce systolic blood pressure by between 5 and 20 mmHg [5]. Alcohol consumption is directly correlated with elevated blood pressure; thus, reducing alcohol intake is associated with lower blood pressure [6]. However, regardless of weight loss, engaging in at least 150 minutes of physical activity per week has been shown to reduce the incidence of T2D by 44% [7]. Furthermore, there are correlations among certain lifestyles, suggesting that individuals who engage in intensive exercise may be more inclined to quit smoking or drinking. Therefore, it is necessary to dynamically analyze the changes in various types of lifestyle modifications rather than focusing on a single type. Furthermore, lifestyle habits such as smoking, drinking, and exercise are influenced by gender. Typically, men are more likely to smoke and drink, leading to the hypothesis that the impact of rigorous management of unhealthy lifestyles on the occurrence of cardiovascular events varies between genders. The frequency and intensity of exercise also differ by gender.
Thus, the present study investigated (1) how dynamic changes in lifestyle can affect cardiovascular events; (2) how various lifestyles change organically; (3) whether gender-specific lifestyle changes can affect the occurrence of cardiovascular events, specifically in relation to prehypertension or prediabetes.
Methods
The Institutional Review Board (IRB) at Ewha Womans Medical College Mokdong Hospital (IRB no. EUMC-2021-11-029) and the NHIS Big Data Steering Department (NHIS-2022-2-197) granted approval for this study. As the NHIS data used were completely anonymous and handled in accordance with the Personal Data Protection Act, obtaining written consent from subjects was not required.
This is a nationwide, population-based cohort study. It has been described in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement, which is available at: https://www.strobe-statement.org/.
In December 2022, the authors conducted a search on prediabetes and prehypertension within the National Health Insurance Service–Health Screening Cohort (NHIS-HEALS) database. Prediabetes is defined by the presence of impaired fasting glucose, impaired glucose tolerance, or a hemoglobin A1c level of 5.7%–6.4%, while prehypertension is characterized by a systolic pressure of 120 to 139 mmHg or a diastolic pressure of 80 to 89 mmHg at the initial health screening. The NHIS-HEALS database was established from a cohort of 514,866 Koreans, aged 40 to 79 in 2002, who were randomly selected to represent 10% of the national health screening subjects from 2002 to 2003.
The source of data for this study was the Korean NHIS-HEALS, which is a nationwide population-based cohort. Detailed information on the cohort has already been published elsewhere [8]. Additional details on the study cohort can be found in the Supplement 1.
For this study, we constructed a sub-cohort based on the research hypothesis. Initially, we excluded subjects with inconsistent examination dates, resulting in a cohort of 514,795 individuals who had undergone at least one health screening between 2002 and 2003. Of these, 334,937 subjects who also participated in a health screening during the second period (2004–2005) were included. We designated the date of the second health screening as the index date (Fig. 1C). Subsequently, we excluded individuals with a history of specific conditions prior to the index date, as defined by the International Classification of Diseases, Tenth Revision (ICD-10): heart failure (ICD-10: I50, n=668); cerebrovascular accident (I60-I69, n=13,187); cardiovascular disease (CVD; I20-I25, I71, I72, n=25,528); or cancer (C00-C96, n=3,921). Additionally, we excluded individuals with missing lifestyle-related data (n=13; n=297,010).
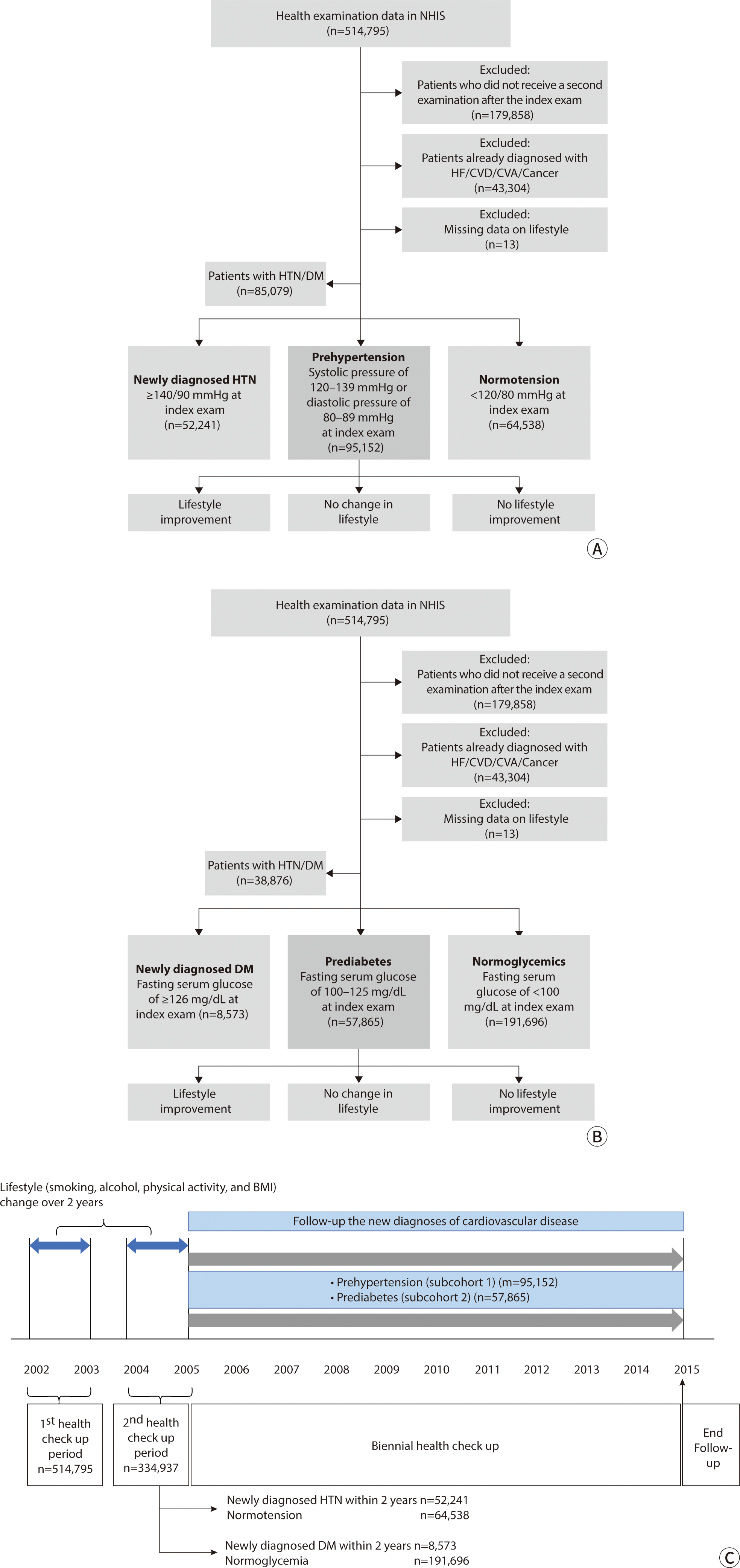
Next, we defined cohorts for prediabetes and prehypertension. Detailed criteria used to define these cohorts are available in the Supplement 1. The prehypertension cohort included 95,152 subjects (60,084 men and 35,068 women), while the prediabetes cohort comprised 57,865 subjects (37,836 men and 20,029 women). The flow diagram illustrating the selection of the study population is displayed in Fig. 1A, B.
The primary outcome of the study was major adverse cardiovascular events (MACE), which were defined as death, non-fatal myocardial infarction (MI), or non-fatal stroke resulting from CVD. Hospital admission data were used to identify instances of CVD, including MI (ICD-10: I21), stroke (ICD-10: I60–I69), and cardiovascular death (I20–I25, I71–I72, and I60–I69). The secondary outcome of the study was all-cause mortality. The date of death was obtained from the claims database, and this information was used to determine all-cause mortality. The follow-up period began on the index date and concluded either on the date of the first occurrence of the primary outcome or on the last follow-up date (12/31/2015).
Regarding lifestyle factors, we considered body mass index (BMI), current smoking status, alcohol intake categorized by weekly frequency (never; 2–3 times a month; 1–2 times a week; 3–4 times a week; ≥5 times a week), and physical activity also categorized by weekly frequency (never; 1–2 times a week; 3–4 times a week; 5–6 times a week; every day). Changes in these lifestyle factors were assessed using health checkup data from the first observation period (2002–2003) to the second (2004–2005) as shown in Fig. 1C. Detailed methods for evaluating changes in lifestyle factors are available in the Supplement 1.
Since subjects were selected from the cohort database according to the inclusion criteria and disease criteria, selection bias was not a concern.
Summary statistics for baseline characteristics were presented as means with SDs for numerical data and as counts with percentages for categorical data. To explore the impact of disease incidence and lifestyle behaviors according to gender, we performed gender-stratified analyses. The incidence rate (per 10,000 person-years) of outcomes was estimated using a Poisson regression model. To evaluate the influence of lifestyle changes on disease incidence, we calculated hazard ratios (HRs) with 95% CIs using the Cox proportional hazards regression model. Detailed descriptions of the covariates used for adjusting the HRs, as well as the methodology for the sensitivity analysis, are available in the Supplement 1.
All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was set to P<0.05.
Results
Males constituted 65.2% of the prehypertension cohort (n=60,084). Additionally, women were found to consume less alcohol (32.0% vs. 80.7% for never drinking) and to exercise less frequently (46.4% vs. 65.0% for never exercising) compared to men. Among the men, 44.1% were current smokers (n=25,201), whereas only 1.7% of women smoked currently (n=577). Similarly, in the prediabetes cohort, men made up 65.4% of the subjects (n=37,836). The percentage of women who neither exercised (47.2% vs. 67.7%) nor consumed alcohol (29.8% vs. 80.9%) was lower compared to men. In this cohort, 41.8% of men (n=15,144) were current smokers, while only 2.2% of women (n=432) were smokers. Furthermore, the prevalence of BMI over 25.0 kg/m2 was lower in women than in men in both cohorts (32.3% vs. 28.5% in the prehypertension cohort and 38.1% vs. 36.7% in the prediabetes cohort). The general characteristics of subjects according to group are summarized in Table 1.
Table 2 presents the incidence of diseases during follow-up in subjects with prehypertension and prediabetes, categorized by gender. In the prehypertension cohort, there were 3,979 MACE cases in men and 2,290 in women. The MACE incidence rates were similar between genders (62.6/10,000 PY for men vs. 62.1/10,000 PY for women; P=0.724). However, a significant difference was observed in all-cause mortality rates, with men showing a higher rate than women (47.1/10,000 PY vs. 24.3/10,000 PY; P<0.001). In the prediabetes cohort, 3,435 MACE cases were recorded in men and 1,904 in women. The incidence rate of MACE in women was slightly higher than in men, but this difference was not statistically significant (88.0/10,000 PY for women vs. 92.8/10,000 PY for men; P=0.063). However, there was a significant difference in all-cause mortality between men and women, with men experiencing a higher rate (66.7/10,000 PY vs. 41.4/10,000 PY; P<0.001).
As previously mentioned, lifestyle factors were defined as BMI, current smoking, drinking, and physical activity. In the prehypertension and prediabetes cohorts, 26.6% and 26.1% of subjects, respectively, experienced a worsening in one or more lifestyle factors. Conversely, 34.2% and 34.8% of subjects in these cohorts improved in one or more lifestyle factors. Overall, fewer than 10% of subjects experienced a decline or improvement in two or more lifestyle factors (Supplement 2).
By controlling for lifestyle and clinical factors at the initial health screening, we evaluated the effects of lifestyle changes on major outcomes using a multivariate model (Tables 3, 4). In the prehypertension group, 7,376 patients (7.75%) had already been diagnosed with T2D, and 2,601 (2.73%) were taking anti-hyperglycemic medication. Similarly, in the prediabetes group, 11,577 patients (20.01%) had been diagnosed with HTN, and 8,962 (15.49%) were on antihypertensive medication (Table 1). To isolate the effects of medication, the multivariate analysis accounted for the impact of antihypertensive drugs, anti-hyperglycemic drugs, and aspirin. In men with prehypertension, the risk of MACE increased if their lifestyle worsened (HR, 1.13; 95% CI, 1.04–1.23, P=0.004), particularly if they gained weight (HR, 1.15; 95% CI, 1.03–1.28, P=0.010) or started smoking (HR, 1.34; 95% CI, 1.17–1.55, P<0.001). For women with prehypertension, the risk of MACE was higher for those who started smoking (HR, 1.69; 95% CI, 1.15–2.49, P=0.008) or reduced their physical activity (HR, 1.25; 95% CI, 1.06–1.47, P=0.010). Conversely, in men with prehypertension, improving lifestyle factors reduced the risk of MACE (HR, 0.91; 95% CI, 0.84–0.99, P=0.025), particularly through smoking cessation (HR, 0.79; 95% CI, 0.70–0.89, P<0.001), drinking less (HR, 1.09; 95% CI, 1.00–1.20, P=0.048), or increasing physical activity (HR, 0.91; 95% CI, 0.84–0.99, P=0.027). In men with prediabetes, those whose lifestyle factors worsened had a 23% higher risk of MACE compared to those with no lifestyle changes (HR, 1.23; 95% CI, 1.12–1.35, P<0.001). An increased risk of MACE was also observed in those who gained weight (HR, 1.19; 95% CI, 1.06–1.33, P=0.003), started smoking (HR, 1.41; 95% CI, 1.22–1.64, P<0.001), or decreased their physical activity (HR, 1.21; 95% CI, 1.09–1.35, P<0.001). Additionally, in men with prediabetes, a reduction in alcohol consumption was linked to a higher risk of MACE (HR, 1.17; 95% CI, 1.07–1.29, P=0.001). In women with prediabetes, the risk of MACE was 1.24 times higher for those who gained weight compared to those with no change in BMI levels (HR, 1.24; 95% CI, 1.06–1.45, P=0.006). As weight change can be a consequence of lifestyle changes, the association between unhealthy lifestyles, excluding BMI, and MACE was evaluated. Among pre-hypertensive men, those whose lifestyles worsened had a higher risk of MACE (HR, 1.10; 95% CI, 1.02–1.20, P=0.022). Among pre-hypertensive women, those whose lifestyles improved tended to have a lower MACE risk, although this association was not statistically significant (HR, 0.91; 95% CI, 0.81–1.01, P=0.072) (Table 3, Supplement 3). In the prediabetes group, men whose lifestyles worsened showed a significantly higher risk of MACE (HR, 1.23; 95% CI, 1.12–1.35, P<0.001), while there was no significant difference in MACE risk among comparative female subjects (Table 4, Supplement 4).
Adjusted covariates include age, income level (quantiles), current smoking status, alcohol consumption, physical activity, CCI score, BMI, systolic blood pressure, total cholesterol, fasting serum glucose level at the first health screening, and usage of statin medication prior to the index date.
Adjusted covariates include age, income level (quantiles), current smoking status, alcohol consumption, physical activity, CCI score, BMI, systolic blood pressure, total cholesterol, fasting serum glucose level at the first health screening, and usage of statin medication prior to the index date. MACE, major adverse cardiovascular events; BMI, body mass index; MI, myocardial infarction; CVD, cardiovascular disease.
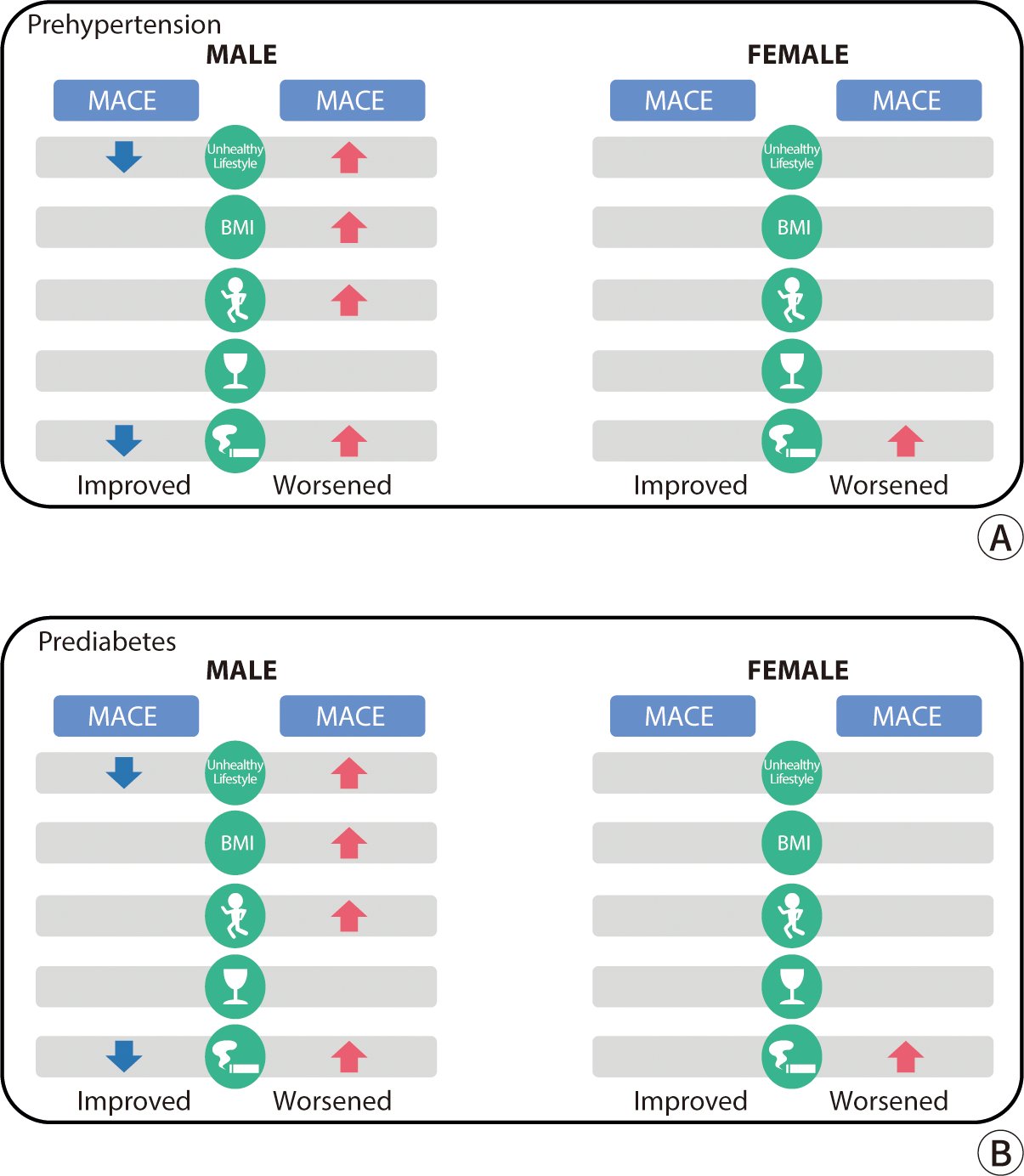
To mitigate the risk of reverse causality, a sensitivity analysis was conducted by excluding cardiovascular events that occurred within two years following the observation of lifestyle changes. In the prehypertension male group, the risk of MACE increased among subjects who experienced a decline in lifestyle quality, gained weight, decreased their physical activity frequency, or began smoking between biennial screenings. Conversely, the risk decreased in those who improved their lifestyles or quit smoking. In the prehypertension female group, an increase in MACE risk was observed in subjects who started smoking (Fig. 2A). In the prediabetes group, the MACE risk escalated in men who worsened their lifestyle, gained weight, reduced their physical activity frequency, or started smoking between the biennial screenings. In women, the risk increased among those who gained weight (Fig. 2B).
Discussion
This study highlights two significant findings: First, the risk of MACE increased in men with prediabetes or prehypertension whose lifestyle factors deteriorated, and this risk escalated even with the worsening of just one parameter. Second, the impact on MACE risk varied slightly depending on lifestyle changes, with smoking being strongly linked to an increased risk of MACE in both prediabetes and prehypertension conditions, irrespective of gender.
Smoking is a well-known risk factor for CVD risk. However, the precise level of risk that smoking presents to CVD in the prehypertensive population has not been clearly defined. The incidence of CVD is higher in people with a blood pressure of 120–129/80–84 mmHg and 130–139/85–89 mmHg than in normal blood pressure group in Europe and the United States [9]. Previous studies have demonstrated that smoking significantly influences the progression from prehypertension to hypertension by stiffening the arteries [10]. Furthermore, smoking increases the risk of developing hypertension in a dose-response manner over long-term follow-up [11]. This study confirmed that MACE increased as smoking habits worsened in both women and men. In prehypertensive patients, smoking not only contributes to the progression to hypertension but also independently elevates the risk of CVD. These findings underscore the importance of smoking cessation in patient education for individuals with prehypertension.
In a previous study, smoking was strongly associated with prediabetes in healthy young individuals, showing a linear risk gradient with increased cumulative smoking exposure [12]. Additionally, prior epidemiological studies have indicated that smoking may be an independent risk factor for T2D and is significantly associated with a higher risk of coronary heart disease [13–16]. A meta-analysis of prospective cohort studies revealed a relative risk of 1.55 (95% CI 1.46–1.64) for total mortality and 1.29 (95% CI 1.29–1.71) for cardiovascular mortality, assessing the relationship between smoking and mortality risk in diabetes patients [17]. In this study, men with prediabetes who started smoking showed an increased risk of MACE, while smoking cessation decreased CVD death.
In this study, we also analyzed changes in the risk of MACE, specifically excluding BMI to assess whether its inclusion as a factor influenced the outcomes. Males in the prehypertension and prediabetes groups who experienced a deterioration in their lifestyle had an increased risk of MACE, a trend that persisted even when BMI was excluded. However, among women in both groups, the risk of MACE did not show significant differences when unhealthy lifestyle parameters were considered, with or without BMI (Tables 3, 4). Previous research has demonstrated a U-shaped relationship between obesity and all-cause mortality and a linear association between BMI and cardiovascular events [18–20]. In this context, changes in an individual's BMI category do not directly correlate with an increase or decrease in CVD risk. For instance, overweight patients with stable coronary heart disease exhibited lower mortality compared to those of normal weight, and overweight patients with acute coronary syndrome showed significantly lower in-hospital and 12-month mortality [21–23]. Furthermore, BMI does not accurately reflect body composition, such as muscle mass or fat distribution. This phenomenon, known as 'reverse causality,' suggests that BMI is often considered a result influenced by other confounding factors rather than a standalone factor in previous studies [24]. In particular, the BMI values in this dataset do not indicate any intention to lose weight. Additionally, a decrease in BMI could also result in a reduction in muscle mass. Given these considerations, although the primary outcome based on lifestyle changes, excluding BMI, revealed significant differences in MACE risk among male groups, an analysis of the impact of BMI parameters on the detailed elements of MACE yielded conflicting results (Supplementals 5, 6).
Previous studies have shown that women generally have lower rates of smoking and obesity than men, while men are more likely to engage in regular and sustained physical activity [25,26]. When comparing men and women, the increase in MACE among men whose unhealthy lifestyles worsened could be linked to differences in lifestyle patterns between the genders. Indeed, data from the NHIS indicate gender-specific lifestyle patterns; men are more likely to smoke, be obese, and consume more alcohol. A previous study demonstrated that moderate to high physical activity during leisure time was associated with a lower risk of MACE [27]. Specifically, Fig. 2 illustrates that a decreased frequency of physical activity was associated with an increased risk of MACE in both the prehypertension and prediabetes male groups.
This study may have several limitations. First, there is a potential for misclassification bias, as the diagnoses of prediabetes and prehypertension were based on the recorded history in the NHIS and single measurements of blood pressure or serum glucose levels. Second, the data on lifestyle patterns were derived from self-reported questionnaires, which may have introduced some misclassification. Third, further research is necessary to determine if similar trends are observable in multi-racial populations beyond Asians. Fourth, the study assessed changes in lifestyle by analyzing data collected at two distinct time points, specifically between the index examination and the second examination. However, it is possible that any lifestyle changes occurring after the second examination were not captured.
In this retrospective cohort study, a worsening smoking habit was associated with an increased incidence of MACE in populations with prehypertension and men with prediabetes. This finding remained consistent after sensitivity analysis. Specifically, a decline in healthy lifestyle habits among men significantly increased the risk of MACE. Therefore, lifestyle modifications are crucial even before the diagnosis of hypertension or T2D, as they can significantly reduce cardiovascular events and mortality, particularly in men.
