Introduction
Infectious spondylitis is a disease that affects the vertebral body, intervertebral disc, or surrounding tissues. Although the site of infection can define the condition, terms such as infectious spondylitis, spondylodiscitis, and vertebral osteomyelitis are often used interchangeably. The causative microorganisms are diverse, varying by region and over time. Most bacteria elicit a pyogenic response, while mycobacteria, fungi, Brucella, and syphilis lead to granulomatous reactions [1]. In Korea, bacteria in general and Mycobacterium tuberculosis in particular are the predominant causes, corresponding to classifications of pyogenic spondylitis and tuberculous spondylitis.
The diagnosis of infectious spondylitis primarily relies on a high level of clinical suspicion, informed by symptoms such as back pain and fever. However, early identification remains challenging, with diagnosis typically taking 1 to 3 months [2,3]. This delay complicates disease management. Infectious spondylitis places a considerable burden on individuals and society, affecting health, economic stability, and quality of life.
This review is designed to provide healthcare professionals with critical insights into the clinical management and treatment of infectious spondylitis. The article thoroughly examines key aspects of this condition within the Korean context, including its prevalence, causative microorganisms, associated comorbidities, diagnostic strategies, therapeutic approaches, and anticipated outcomes. Our goal is to deepen clinicians’ understanding and foster improved patient care in cases of infectious spondylitis.
Ethics statement
It is a literature database-based review; therefore, neither approval by the institutional review board nor obtainment of informed consent was required.
Incidence
The incidence of infectious spondylitis in Korea has varied over time. Prior to the early 2000s, tuberculous spondylitis was believed to predominate, reflecting the high prevalence of tuberculosis [4].
Fig. 1 depicts the incidence of infectious spondylitis, based on national health insurance data from Korea. A nationwide cohort study conducted from 2007 to 2016 identified 9,655 cases of the condition [5]. The findings showed an increase in the number of pyogenic spondylitis cases, risking from 2,431 in 2007 to 4,874 in 2016. Conversely, the incidence of tuberculous spondylitis declined from 1,756 cases to 594 over the same timeframe. These patterns indicate a shift toward bacterial infection as the predominant cause of infectious spondylitis in Korea.
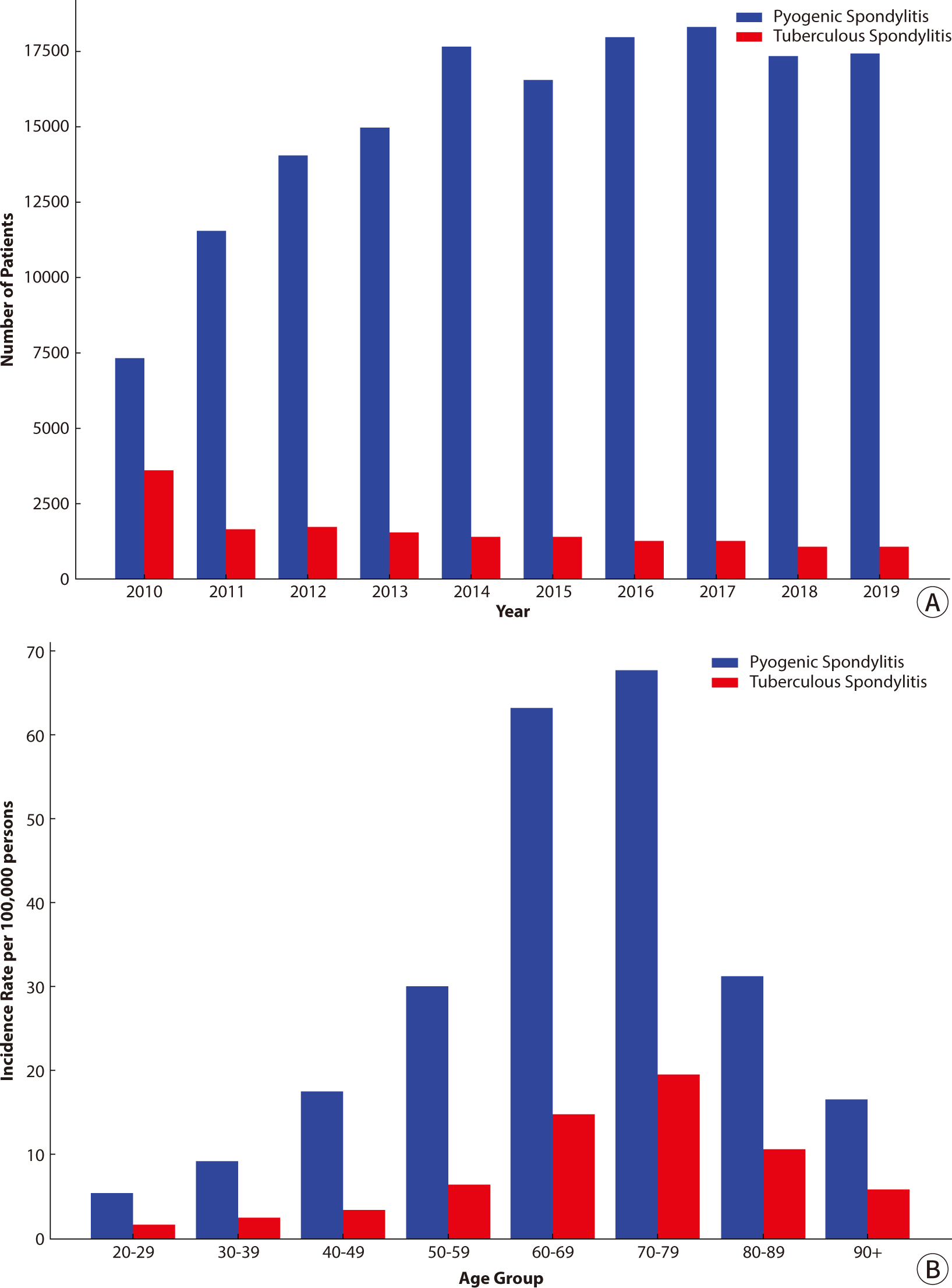
A more recent study covering the period from 2010 to 2019 further confirmed this upward trend (Fig. 1A) [6]. Among 169,244 patients, the number of cases increased from 10,991 in 2010 to 18,533 in 2019. In turn, the incidence rate per 100,000 people climbed from 22.90 to 35.79. This increase is attributed to the aging population, higher prevalence of chronic diseases, increased use of immunosuppressive therapies, and greater frequency of invasive spinal procedures [7–9].
Both pyogenic and tuberculous spondylitis exhibited the highest prevalence in individuals aged 60−79 years (Fig. 1B) [5]. Interestingly, female patients predominated in both groups, which contrasts with some international studies reporting a higher incidence in male patients.
Anatomically, infectious spondylitis predominantly affects the lumbar region, followed by the thoracic and cervical spine, with the latter comprising less than 10% of cases [10]. Pyogenic spondylitis primarily targets the lumbar spine, whereas tuberculous spondylitis more commonly occurs in the thoracic spine, with the lumbar region representing the second most frequent site [3].
Etiologic microorganisms
In Korea, fungal spondylitis, non-tuberculous mycobacteria, and Brucella spondylitis are uncommon [9,11,12]. Microorganisms that cause pyogenic spondylitis typically reach the vertebrae through arterial spread, during spinal surgery or other procedures, or directly from adjacent sites. Staphylococcus aureus is the predominant causative agent in pyogenic spondylitis, followed by Streptococcus species. Gram-negative bacilli are responsible for 7% to 33% of cases, with Escherichia coli being the most common among them [10,13,14]. Coagulase-negative staphylococci are implicated in 30% to 32% of pyogenic spondylitis cases in patients with a history of spinal surgery or other procedures [15]. Gram-negative bacilli are more frequently suspected in female patients or in those with previous or concurrent urinary tract or intra-abdominal infections [10,14]. Table 1 presents the distribution of microorganisms identified in cases of spontaneous or postoperative pyogenic spondylitis based on Korean data [15,16].
Tuberculous spondylitis primarily results from venous spread originating in the lungs or other primary lesions. M. tuberculosis can directly infect the spine from adjacent organs, including the lungs, kidneys, and gastrointestinal tract. A literature review by Schirmer et al. [17] indicated that the rate of concomitant pulmonary tuberculosis in patients with tuberculous spondylitis ranges from 8% to 100%. Additionally, a study from Korea found that 16% of patients with tuberculous spondylitis also had extrapulmonary tuberculosis, including miliary tuberculosis as well as renal and lymph node involvement [18].
Comorbidity with other disease
Understanding the distribution of microorganisms based on patient characteristics can guide clinicians in selecting appropriate empirical antibiotics. An analysis of Health Insurance Review and Assessment Service data from 2010 to 2019 showed that patients with infectious spondylitis often exhibit multiple comorbidities. These include diabetes mellitus (55.1%), rheumatoid arthritis (27.3%), chronic obstructive pulmonary disease (15.2%), and end-stage renal disease (12.8%) [6]. In a cohort of 586 patients with culture-proven pyogenic spondylitis, the most common comorbidities were diabetes (30.7%), solid tumors (14.3%), chronic renal disease (10.4%), and liver cirrhosis (9.4%) [16]. The study also revealed that gram-negative infections were relatively prevalent among older patients, women, and those with cirrhosis or solid tumors. Additionally, methicillin-resistant S. aureus infection was more frequent in patients with chronic renal disease than in those without this comorbidity [16].
While one report indicated that diabetes was reported in 17% of 94 patients with tuberculous spondylitis, no other comorbidities were specifically associated with this condition [3]. In 2020, Korea had the highest incidence of tuberculosis among Organisation for Economic Co-operation and Development countries, with 49 cases per 100,000 population, and an increasing proportion of new cases were seen in individuals aged 65 and older [19]. Consequently, the range of comorbid diseases in patients with tuberculous spondylitis may be diverse.
Diagnostic approaches
Clinicians should consider infectious spondylitis in patients presenting with new or worsening back or neck pain. The onset of symptoms is often gradual and subtle, with pain typically worsening during weight-bearing activities and subsiding when the patient lies down. The pain is usually well-localized and can be reproduced through palpation or percussion over the affected area. Pyogenic spondylitis is relatively likely among patients who experience back or neck pain along with fever, bloodstream infection, or infective endocarditis [20]. This condition should also be suspected in patients presenting with fever and new peripheral neurologic symptoms, with or without back pain. Radiculopathy, which may manifest as leg pain or weakness, can occur due to nerve root compression or irritation. In cases involving the thoracic spine, patients often describe a “belt-like” pain across the chest wall or abdomen, which can be mistakenly attributed to gastrointestinal, cardiac, or pulmonary conditions.
The initial evaluation of patients with suspected infectious spondylitis should begin with a comprehensive history and physical examination, including a detailed neurological assessment. Patients should be asked about any comorbidities, ongoing infections, and predisposing factors, such as existing non-spinal infections, the presence of indwelling devices, recent application of surgical instruments, and spinal injections [10,15]. Initial diagnostic tests include inflammatory markers (WBC count, ESR, and CRP level), as well as two sets of blood cultures. Spinal imaging is also critical, with MRI being the preferred method when available. Additionally, plain X-rays, including anteroposterior and lateral views, along with flexion/extension views, should be obtained for baseline evaluation in all cases [21]. However, native X-rays exhibit low specificity for diagnosing infectious spondylitis, with these examinations primarily detecting advanced cases characterized by vertebral endplate irregularities or a reduction in intervertebral disc height.
MRI with intravenous gadolinium contrast is the preferred imaging method due to its increased sensitivity and specificity. It offers superior visualization of potential infection spread to the epidural and paravertebral spaces [22]. Clinicians should obtain T2-weighted and post-contrast T1-weighted images with fat suppression. Typical MRI findings indicative of infection include abnormal signals from the intervertebral discs, destruction of the vertebral body endplates adjacent to the disc, and bone marrow edema. However, these findings can also be present in non-infectious spinal conditions, necessitating collaboration between clinicians and radiologists to achieve accurate diagnosis and differentiation [22].
For patients unable to undergo MRI, CT can be used to assess the osseous anatomy and, with the addition of contrast, can also reveal involvement of paraspinal and epidural soft tissues. Recent studies have suggested that fluoro-2-deoxyglucose PET/CT may represent a complementary tool to MRI for differentiating between tuberculous and pyogenic spondylitis [23], as well as for assessing disease activity [23,24]. PET/CT offers superior spatial resolution and improved detection of metastatic infection. The combination of positive blood cultures, imaging findings, and clinical symptoms can often confirm a diagnosis of infectious spondylitis [25]. In blood cultures of patients suspected of having pyogenic spondylitis, when microbial growth is present, the necessity of tissue biopsy remains a topic of debate [20]. A Korean retrospective study involving 141 patients with pyogenic spondylitis, who exhibited positive blood and tissue cultures, reported a 95.7% concordance rate in bacterial identification [26]. Discordant results were typically characterized by the growth of a single species in one sample and multiple species, including the initially identified species, in the other. These findings suggest that in cases with positive blood cultures, tissue biopsy may not be necessary for the microbiological diagnosis of pyogenic spondylitis (Fig. 2).
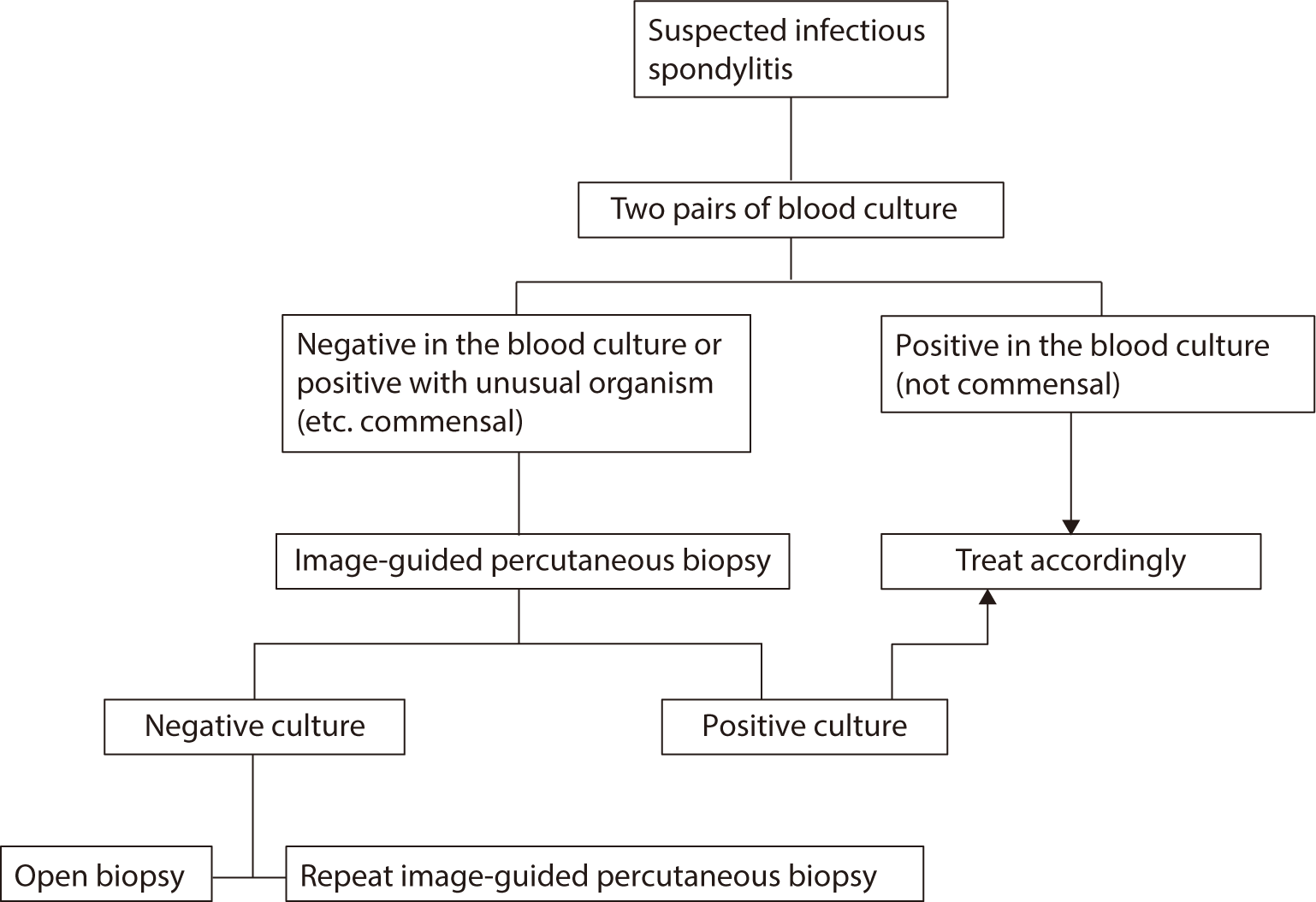
Inflammatory markers such as WBC count, CRP level, and ESR are typically elevated in acute infections but may be normal in chronic cases [26]. Kim et al. [3] found that patients with pyogenic spondylitis exhibited significantly higher levels of ESR and CRP compared to those with tuberculous spondylitis. Notably, tuberculosis infection rates remain high among elderly Koreans, warranting careful consideration in this demographic [18,27]. Tuberculous spondylitis should be suspected in cases involving slow disease progression over several months or when extraspinal tuberculosis is detected [3,18]. Diagnosis is confirmed through tissue biopsy, with mycobacterial culture positivity rates ranging from 69.0% to 85.3% [28]. Polymerase chain reaction techniques have been employed for the rapid identification of mycobacteria in formaldehyde-fixed, paraffin-embedded tissue specimens. Tissue biopsy is also indicated when blood cultures fail to establish a microbiologic diagnosis for pyogenic spondylitis. The two most widely recognized methods are image-guided percutaneous needle biopsy and open biopsy. Once tissue biopsy is performed, specimens should be sent for both microbiologic and histopathologic examination. Needle biopsy specimens can be obtained percutaneously through CT or fluoroscopically guided biopsy, with diagnostic yields of 44% and 55%, respectively [29]. If needle biopsy is indicated for patients with concurrent paraspinal inflammation or abscess, samples should be collected from paraspinal rather than spinal tissues [30]. Open surgical biopsy is considered the most reliable method, with a 76% diagnostic yield according to a recent systematic review [31]; however, the impact of prior antibiotic use requires further clarification. Some experts suggest that in patients with pyogenic spondylitis who have been exposed to antibiotics but show no signs of sepsis or severe sepsis, a certain interval should elapse before biopsy is performed [32].
A second percutaneous biopsy may be warranted if the initial biopsy does not yield a diagnosis, although the precise benefit of this procedure is still uncertain [33]. It is advisable to wait at least 3 days after the initial biopsy before repeating the procedure, by which time most positive cultures from the first biopsy should have been obtained [34]. Alternatively, if the first image-guided biopsy yields a negative result, proceeding with an open biopsy as the next step is reasonable (Fig. 2).
If the microbial etiology is not identified, empiric treatment becomes necessary. Empiric antibiotics should be promptly administered to critically ill patients showing signs of sepsis or those being taken to the operating room for neurologic compromise. The initiation of empiric treatment should be based on the most likely microbial etiology. To select the appropriate empiric antibiotics for a patient with pyogenic spondylitis of unknown microbial etiology, factors such as medical history, demographic characteristics, clinical features, and imaging results must be considered [4,16]. If the patient has not undergone spinal surgery, vancomycin need not be included in the empiric antibiotic regimen due to the low risk of methicillin-resistant S. aureus or methicillin-resistant coagulase-negative staphylococci [13,15,35]. A first-generation cephalosporin is suitable for the treatment of suspected community-acquired pyogenic spondylitis. Alternative options include a fluoroquinolone with rifampin, or a fluoroquinolone plus a beta-lactam/beta-lactamase inhibitor [36,37]. If the patient has exhibited previous or concurrent urinary tract infection or intra-abdominal infection, empiric antibiotics should provide coverage for gram-negative bacilli [10]. Therapy should be adjusted according to bacteriologic test results. Most cases of pyogenic spondylitis are treated conservatively, with favorable outcomes. A recent study has established that a 6-week course of systemic antibiotics is sufficient for most cases [38]. However, a longer duration of therapy may be required in certain situations, such as infections with extensive spread to paraspinal soft tissues, undrained paravertebral abscesses, or extensive bone destruction. Transitioning to oral antibiotics with high bioavailability is considered acceptable.
In cases of culture-negative infectious spondylitis, which typically involve long-term and broad-spectrum antibiotic treatment, this strategy can result in avoidable side effects and contribute to antibiotic resistance. One prior report indicated favorable outcomes with the use of cefazolin in hematogenous pyogenic spondylitis and with vancomycin in post-procedural pyogenic spondylitis among patients with culture-negative pyogenic spondylitis [39].
Treatment
The most severe complication of infectious spondylitis is neurologic impairment, which can occur secondary to either abscess formation or bony collapse. Treatment objectives include saving the patient’s life, alleviating pain, preventing or reversing neurologic deficits, eradicating the infection, and restoring spinal stability. To meet these treatment objectives, management principles encompass: (1) establishing an accurate microbiological diagnosis; (2) administering appropriate antimicrobials; (3) immobilizing the spine; and (4) carefully monitoring for clinical and radiographic evidence of spinal instability, as well as for progression of the infection or neurological deterioration.
The treatment regimens for tuberculous spondylitis align with those for pulmonary tuberculosis. For most patients receiving rifampin for susceptible tuberculosis, a 6- to 9-month course of therapy is sufficient [40]. To date, no formal data are available on the efficacy of newer drugs in the treatment of osteoarticular tuberculosis.
While receiving antimicrobial therapy, patients should be carefully monitored for clinical signs of soft tissue extension or abscess, as well as for symptoms of cord compression. Additionally, clinicians should track inflammatory markers, specifically ESR and CRP levels, with weekly assessments [20]. CRP levels tend to normalize more quickly than ESR following successful treatment or after uncomplicated spinal fusion surgery [41]. Routine anteroposterior and lateral radiographs centered on the affected disc are recommended at 1 and 3 months into antimicrobial therapy, and again 3 months after the cessation of treatment [42]. For the cervical or lumbar spine, orthopedic surgeons advise obtaining follow-up flexion/extension films to reliably detect potential instability or to confirm bone fusion. In patients who are clinically improving while on treatment, routine follow-up MRI is unnecessary, as imaging findings may not correspond with clinical progress [43].
Surgical intervention, which may include procedures such as incision and drainage, decompression, corpectomy, and fusion, is sometimes required. Patients presenting with neurological deficits such as weakness, paresthesia, and urinary retention, as well as those with radiographic signs of epidural or paravertebral abscess or actual or impending spinal cord compression, should be evaluated for surgical decompression. Interventional radiology has become increasingly important in managing psoas muscle abscesses. Continuous monitoring for the development or progression of neurological signs is crucial, yet it is frequently overlooked. Epidural abscesses can lead to abrupt neurological deficits. A spinal epidural abscess, a potentially severe complication of infectious spondylitis, can spread through septic thrombosis of the epidural veins. Since skip lesions, or noncontiguous abscesses, may occur in 15% of overall cases [44], imaging of the entire spine is recommended.
Relative indications for surgery include uncertain diagnosis, lack of clinical improvement following antimicrobial treatment, or significant progressive spinal deformity accompanied by biomechanical instability. However, guidelines do not offer a detailed and practical description of surgical interventions for cases of spondylitis that are resistant to conservative treatment [20]. Decisions regarding surgery should be made in close consultation with surgeons.
In the early phase of infectious spondylitis, bed rest is recommended until the acute pain improves. Both bed rest and spinal immobilization are crucial, particularly in cases of vertebral destruction. Once the acute pain has subsided, ambulation with an appropriate brace is advised. Patients with thoracic infections should use a thoracolumbar sacral orthosis, while those with lumbosacral infections are advised to use a lumbar sacral orthosis. The duration of thoracolumbar sacral orthosis brace usage varies depending on factors such as the patient’s response to treatment, the nature of the infection, and the overall health and stability of the spine. Research indicates that approximately 30% of patients may experience a progression of deformity during the first 6 to 8 weeks [45]. Typically, patients may need to wear the brace continuously for several weeks to months, with the duration of use gradually decreasing as healing progresses. Patients should be monitored throughout the treatment and for 1 year after its completion to detect any relapses [46].
Prognosis
Most patients experience a gradual improvement in back pain after the initiation of treatment, with the pain typically resolving after bone fusion occurs. However, clinicians must communicate to patients and their caregivers that back pain may persist. A systematic review of the clinical characteristics of infectious spondylitis reported an attributable mortality rate of 6% [47]. The functional outcome is worse in cases with neurological deficits, which have been noted in 32% of patients. Additionally, 27% of patients experience complications that significantly impact their quality of life [47]. In a large retrospective study from Japan, which included over 7,000 patients with infectious spondylitis, the in-hospital mortality rate was 6% [48]. Comorbidities such as diabetes, end-stage kidney disease, cirrhosis, malignancy, and infective endocarditis were determinants of this mortality rate. Similarly, a retrospective study from a single center in Korea, which included 116 patients with infectious spondylitis, reported an in-hospital mortality rate of 6% and a relapse rate of 8% [9]. Recurrences typically occur within 6 months, and rarely up to 1 year, after the completion of antibiotic therapy [35].
Conclusion
Infectious spondylitis is a serious condition that necessitates timely diagnosis and effective treatment to reduce the risk of complications, such as neurological impairment. The incidence of pyogenic spondylitis has risen in Korea, while tuberculous spondylitis remains a key concern due to the persistent prevalence of tuberculosis. Accurate microbiological diagnosis, appropriate antimicrobial therapy, and vigilant monitoring are essential for the management of infectious spondylitis. Both medical and surgical interventions are important and are chosen based on the severity and progression of the disease. Clinicians must recognize the variety of etiological microorganisms, consider patient comorbidities, and understand the vital role of a multidisciplinary approach in delivering optimal care. Ongoing education and research are imperative to establish standardized treatment protocols and improve prognoses for patients with infectious spondylitis.

