Introduction
Local ischemic preconditioning (IPC) is a phenomenon where brief, repeated cycles of ischemia and reperfusion protect against a subsequent, prolonged period of potentially lethal ischemia in the same tissue or organ [1,2]. Despite substantial supportive experimental data, its clinical application has been limited due to the challenges of inducing ischemia in the target organ, such as the heart, before a predictable insult. Remote IPC (rIPC) is a newer method where transient ischemia and reperfusion of one tissue or organ provide protection against a subsequent, prolonged period of ischemia in a distant organ [3]. For instance, we have demonstrated that transient limb ischemia can protect the heart as effectively as IPC in experimental models [4] and can reduce myocardial damage and enhance lung function in children undergoing heart surgery [5]. This method's advantage lies in its simplicity and potential to protect multiple organs without needing to induce ischemia in the target organs, which could lead to dysfunction. Similarly, volatile anesthetics are recognized for their protective effects against ischemia-reperfusion injury across multiple organs, akin to IPC, and are therefore often referred to as “anesthetic-induced preconditioning” (APC) [6]. Although research into the signaling pathways involved in both rIPC and APC is ongoing [7,8], no studies have yet investigated the potential interactions between rIPC induced by transient limb ischemia and APC. As a result, it remains unclear whether using these two clinically viable techniques together might be complementary, additive, synergistic, or adverse.
Methods
The study protocol was approved by the local committee of the Animal Care and Use of Ewha Womans University College of Medicine (No. SK-2006-6377). The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by NIH (NIH publication N0. 85-23, revised 1996).
Male New Zealand White rabbits weighing 3–4 kg were utilized in this study. During either sham operations or preconditioning, the rabbits were anesthetized with an intravenous injection of Akmezine—a mixture containing 1.18 mg of acepromazine, 0.8 mg of atropine, and 58.81 mg of ketamine per mL of sterile saline—at a dosage of 0.25 mL/kg of body weight initially, followed by intermittent doses of 0.2–0.3 mL depending on the response to painful stimuli. They were allowed to breathe spontaneously, receiving a mixture of oxygen and medical air via a face mask. The animals were randomly assigned to one of four groups: control, rIPC, APC, and rIPC+APC, with six rabbits in each group. The procedures each group underwent are summarized in Fig. 1. The control group experienced the same duration of sedation as the others but did not receive any preconditioning stimulus. The rIPC group underwent four cycles of 5-minute ischemia on a hind limb followed by 5 minutes of reperfusion. The cessation of limb flow following tourniquet application for rIPC was confirmed using pulse oximetry on the affected limb. The APC group was administered 2.5 vol% sevoflurane for 20 minutes via a face mask, followed by a 20-minute washout period. The end-tidal concentration of sevoflurane was monitored using a multi-gas monitor attached to the expiratory limb of the anesthesia circuit. The rIPC+APC group received both rIPC and APC simultaneously, employing the techniques described above.
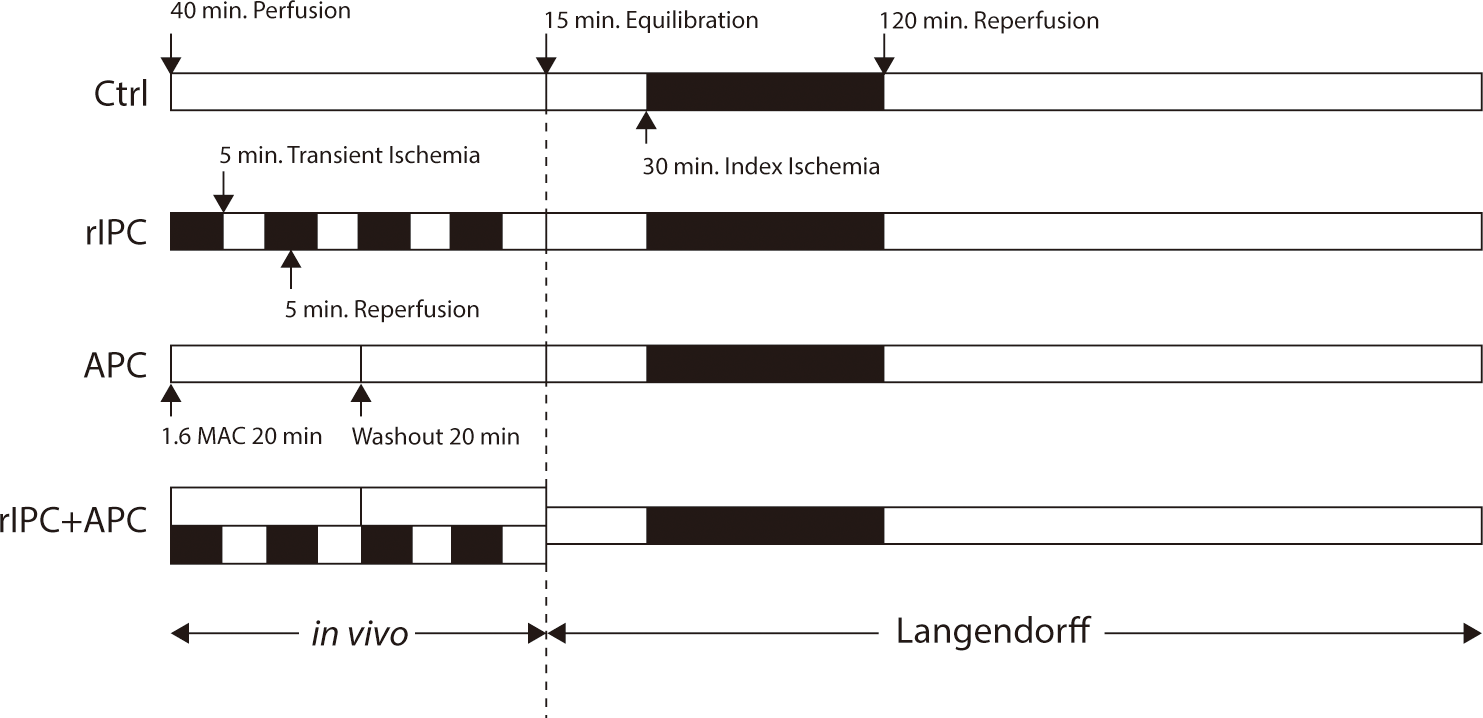
Fifteen minutes after either a sham operation or preconditioning, systemic anticoagulation was achieved with an intravenous injection of heparin (1,000 U/kg of body weight). Once adequate anesthesia and analgesia were confirmed, the hearts were excised and immediately mounted on a recirculating Langendorff apparatus. The hearts were perfused retrogradely at a constant flow rate of 25–26 mL/min using a modified Krebs-Henseleit buffer. The buffer's composition per liter was as follows: NaCl 118 mM, KH2PO4 1.2 mM, KCl 4.7 mM, MgSO4 1.2 mM, CaCl2 1.8 mM, NaHCO3 25 mM, and glucose 10 mM. The perfusate was continuously bubbled with a gas mixture of 95% O2 and 5% CO2 and maintained at a temperature of 37°C using a heat exchanger and warming pump. The heart rate was kept above 180/min, achieved by pacing the right atrium throughout the stabilization period and the 2-hour reperfusion following ischemia. After a 15-minute stabilization period, the hearts underwent 30 minutes of global zero-flow ischemia. During this time, the hearts were immersed in a water-jacketed chamber, also maintained at 37°C, and allowed to beat spontaneously. Following the ischemic period, the hearts were reperfused for 2 hours, and pacing of the right atrium was resumed. The frequency of reperfusion arrhythmias was recorded. While ventricular fibrillation typically reverted to sinus rhythm either spontaneously or in response to physical stimulation, it was incessant in 4 hearts, which were subsequently excluded from the analysis.
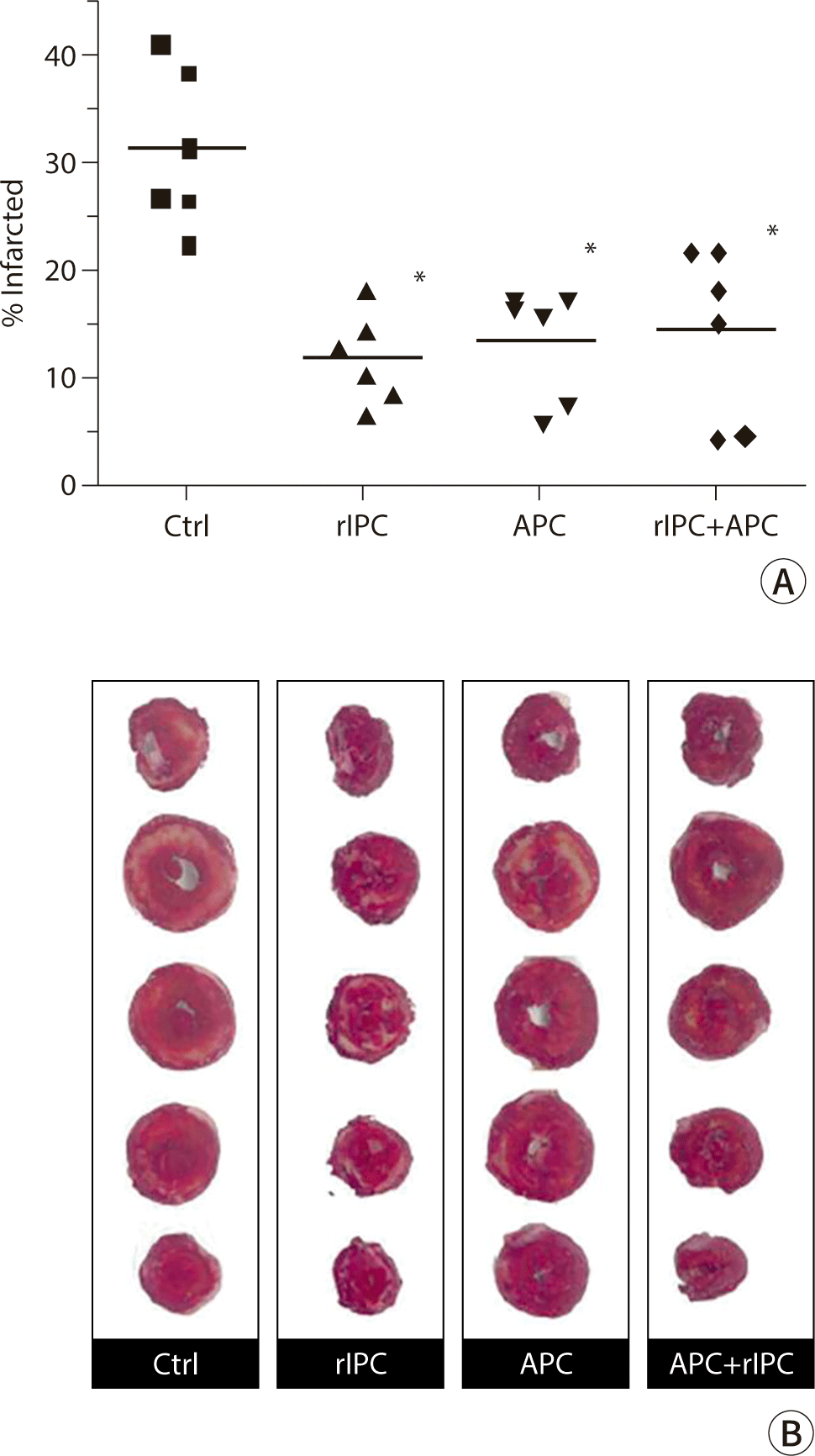
At the end of the reperfusion period, hearts were removed from the Langendorff apparatus, and the left ventricle (LV) was carefully dissected from the rest of the heart. The LV was then transversely sectioned into six slices, each 2–3 mm thick, perpendicular to the septum from the apex to the base. Each slice was incubated in 2, 3, 5-triphenyltetrazolium chloride (TTC, 1% in 0.1 M phosphate buffer, pH 7.4, Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 30 minutes and subsequently fixed in 4% formaldehyde. The slices were then placed on a translucent acrylic holder, and both sides of each slice were photographed using a scanner. The images were analyzed using computerized planimetry (Adobe Photoshop® software, Adobe Systems Incorporated, Mountain View, CA, USA) to determine the area of infarction as a percentage of the total cross-sectional area of the LV. The percentage of infarction for each heart was calculated using the formula: [(A1 × W1) + (A2 × W2) + (A3 × W3) + (A4 × W4) + (A5 × W5) + (A6 × W6)] ×100 / Weight of heart, where Ai is the mean area of infarct for each slice and Wi is the weight of the respective section.
Values are expressed as mean±SEM unless stated otherwise. One-way analysis of variance (ANOVA) followed by a post hoc test was used to compare infarction sizes. Categorical variables were analyzed using Fisher’s exact test where appropriate. The null hypothesis was rejected at P<0.05.
Results
The body weight was comparable across all groups (control: 3.9±0.11 kg, rIPC: 3.6±0.08 kg, APC: 3.6±0.14 kg, rIPC+APC: 3.6±0.07 kg, P>0.05). Out of 28 hearts mounted on the Langendorff apparatus, four were excluded due to persistent ventricular fibrillation upon reperfusion (control: 2, rIPC: 1, APC: 1, P>0.05).
The infarct size, corrected for weight, was assessed in different treatment groups following global ischemia and reperfusion using the Langendorff apparatus (Fig. 2A). This was determined through TTC staining and planimetry, depicted in Fig. 2B. Both rIPC and APC significantly reduced the size of myocardial infarction by more than 50% compared to the control group. However, combining rIPC and APC did not provide any additional protective effect (rIPC, 12.1±1.7%; APC, 13.5±2.1%; rIPC+APC, 14.4±3.3%; P<0.01 compared to control, 31.3±3.0%). There was no significant difference in infarction size among the rIPC, APC, and rIPC+APC groups (P>0.05).
Discussion
This study demonstrated that sevoflurane and rIPC are equally effective in reducing experimental myocardial damage. Additionally, when administered in combination, these interventions were neither synergistic nor adverse.
It is well known that various inhaled halogenated anesthetic agents can induce a preconditioning-like effect, which has been demonstrated to reduce ischemic myocardial damage in experimental models [9] and in some clinical studies [10,11]. However, the temporal activity of APC varies among different anesthetics, and there are significant uncertainties in the existing literature regarding their activity and potency. In the first study to explore the potential differences among agents known to induce anesthetic preconditioning, halothane, isoflurane, desflurane, and sevoflurane were evaluated using an in vivo rabbit coronary ligation model [9].
The anesthetic protocol was consistent across all groups, involving a 30-minute administration of 1 minimum alveolar concentration (MAC) of each anesthetic, followed by a 15-minute washout period, and then a 30-minute period of circumflex coronary artery occlusion. Halothane, isoflurane, and desflurane were found to be protective, significantly reducing the size of subsequent infarcts. However, sevoflurane did not confer myocardial protection when administered according to their protocol. It has been proposed that the washout period may be a critical factor in the efficacy of sevoflurane in this model. In a previous study [12], administering 1 MAC of sevoflurane for 30 minutes immediately before ischemia reduced infarct size in dogs. However, the same dosage followed by a 30-minute washout period did not yield a reduction in infarct size. Interestingly, introducing an additional 2 minutes of local ischemia during the 30-minute washout period led to a decrease in infarct size, with the level of cardioprotection being comparable to that achieved by sevoflurane alone, without a washout period.
The anesthetic protocol used in the current study differed somewhat from those in earlier studies. We administered a slightly higher dose of sevoflurane (2.5 vol% compared to 1 MAC=2.36 vol% in the canine studies mentioned previously) and induced infarction using a Langendorff model. Despite a washout period of 20 minutes, we successfully demonstrated significant myocardial protection, achieving an approximate 50% reduction in myocardial infarction compared to the control group.
The data on the clinical effectiveness of sevoflurane as a cardioprotective agent are somewhat contradictory. In 2007, Piriou et al. [13] investigated the effects of administering sevoflurane for 15 minutes before cardiopulmonary bypass in patients undergoing coronary artery surgery. This two-center study found no effect on postoperative troponin I levels compared to the control group. This contrasts with the findings of De Hert and colleagues, who reported cardioprotective properties of sevoflurane in patients undergoing similar surgeries [14,15]. In their studies, sevoflurane was administered throughout the cardiopulmonary bypass and appeared to provide a protective effect. However, the cardioprotective benefits were less certain when sevoflurane was administered only before the bypass or only after the completion of the coronary anastomosis.
rIPC is an emerging strategy to prevent myocardial damage during cardiac surgery. The effectiveness of local preconditioning of the heart and other organs has been recognized since it was first described by Murry in 1986 [1]. Although highly effective in experimental models, the requirement to induce ischemia in the target tissue or organ before intervention has limited its clinical use. Remote preconditioning, or “preconditioning at a distance,” was initially described by Przyklenk in 1983 [3]. In the foundational study, preconditioning the territory of the circumflex coronary artery reduced myocardial infarction following prolonged ligation of the left anterior descending coronary artery. Later studies in rodents demonstrated that similar protection could be achieved by transient ischemia of more distant organs, such as the kidney or mesentery [7]. While remote preconditioning is as effective as local preconditioning, it has not been more clinically applicable than its local counterpart. However, transient limb ischemia as a stimulus for remote preconditioning of the heart shows greater potential. This straightforward technique, which employs a standard blood pressure cuff or tourniquet, has proven highly effective in animal models and, more recently, in clinical studies involving children and adults undergoing cardiovascular surgery [5]. Notably, in most published animal studies and in two of the three positive clinical studies, these effects were observed to be additive to those of inhalational halogenated anesthetics [5,16].
In our own randomized trial focusing on remote preconditioning in children undergoing cardiac surgery with cardiopulmonary bypass, sevoflurane was administered only during the induction of anesthesia. This was followed by a fentanyl infusion and the inhalation of isoflurane throughout the procedure [5]. Despite this regimen, remote preconditioning demonstrated additional benefits compared to the control group. Children who underwent remote preconditioning through transient limb ischemia before cardiopulmonary bypass exhibited decreased troponin release, lower inotrope scores, and enhanced lung function relative to controls. In a separate study involving adults scheduled for elective abdominal aortic aneurysm surgery, participants were randomized to undergo a single 10-minute episode of femoral artery occlusion prior to aortic cross-clamping [16]. Although all patients received general anesthesia with inhaled desflurane for the duration of the operation, those in the preconditioned group experienced a highly significant reduction in the incidence of postoperative renal dysfunction and myocardial infarction.
Our study was not designed to explore mechanistic differences in comparison to other studies; however, the marked variability in the effects of sevoflurane could stem from relatively minor methodological differences in experimental preparations.
Further studies are needed to determine if there is a dose-response effect for either intervention, whether used alone or in combination. However, we can confirm that there is no inhibitory effect of one intervention on the other when co-administered in this manner. This information will be valuable in designing future clinical studies that involve both techniques. Clearly, a more rigorous assessment of their relative potencies and potential interactions should be addressed in future experimental studies.
The previous clinical studies do not clarify whether the combination of inhaled anesthesia and remote preconditioning has an additive, neutral, or detrimental effect on optimal myocardial protection. Therefore, the current study is timely in assessing this potential interaction. The level of protection provided by sevoflurane was comparable to that achieved through remote preconditioning via transient limb ischemia. Notably, when both were administered together, the degree of protection remained unchanged.

