Introduction
According to the 2020 World Drug Report [1], the number of deaths due to opioid overdose has increased by 2.5 times, rising from 18,515 in 2007 to approximately 47,000 in 2018. A significant aspect of the current overdose crisis is the growing addiction to prescription narcotics [2]. Among patients prescribed narcotics for chronic pain, 21%–29% misuse them, and 8%–12% develop an addiction to these drugs [3]. In recent years, there has been a growing public awareness of the severity of addiction to prescription narcotics [4].
Narcotic abuse can be defined in various ways; for example, MedlinePlus defines prescription narcotic abuse as "taking medicine in a way that is different from what the doctor prescribed" [5]. The primary cause of narcotics abuse is often a strong desire to obtain more narcotics than those prescribed by doctors, driven by a "strong desire or urge to use the substance" [6]. To combat prescription narcotic use disorder (NUD), the Centers for Disease Control and Prevention (CDC) in the United States has issued guidelines for prescribing narcotics [7]. Most states now mandate the registration of narcotic users through prescription drug monitoring programs (PDMPs), which track narcotic prescriptions [8]. According to the CDC guidelines, prescribing a morphine milligram equivalent (MME) per day of ≥90 as the morphine equivalent daily dose (MEDD) should be minimized as much as possible [7]. Some studies have indicated that this system has decreased the prevalence of NUD and its sequelae [9,10], but a recent review has reported ambiguous results [11].
A limitation of the CDC guideline and PDMP is that they can only detect the risk of NUD, not NUD itself. Furthermore, the cut-off values for the NUD high-risk indexes are not definitive standards for identifying patients at risk of NUD. For instance, the MEDD restriction cut-off varies significantly between countries: it is 90 MME/day in the USA and 200 MME/day in Canada [12]. Although numerous studies have sought to develop tools to predict NUD, these tools primarily focus on analyzing high-risk factors rather than detecting NUD itself [13,14]. Therefore, there is a need to develop a method that can definitively identify abnormal prescription patterns indicative of NUD.
Hence, in this study, we developed a method to screen for patients with NUD by directly applying the definition of prescription narcotic abuse to analyze extensive real-world clinical data. Patients employing multiple strategies to obtain additional narcotics may have an actual MEDD that exceeds the doctor's intended MEDD due to overlapping prescriptions. To address this, we introduced a weighted (wt)-MEDD score. This score is calculated based on the number of prescription dates where the MEDD ratio ([actual MEDD]/[intended MEDD]) was above a certain level (for example, 1.5), suggesting the presence of NUD, as per the criteria for prescription narcotics abuse.
Methods
This study received approval from the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 1806-182-955) and adhered to the ethical standards established in the 1964 Declaration of Helsinki. The requirement for informed consent was waived because the study was based on database analysis.
This retrospective cohort study employed real-world data to develop detection methods based on a defined criterion for narcotic abuse. The study adhered to the guidelines outlined in the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement, which can be accessed at: https://www.strobe-statement.org/.
This study evaluated the total narcotics prescriptions from July 2000 to June 2018 at a single large hospital. Prescriptions for patients with cancer and for inpatients were excluded from the analysis. The analysis focused on the following 12 narcotics: fentanyl, hydrocodone, hydromorphone, morphine, oxycodone, oxycodone/naloxone, tapentadol, alfentanil, meperidine, remifentanil, buprenorphine, and nalbuphine. Low-dose narcotics such as codeine and tramadol were excluded from the study.
Most patterns of NUD involve taking higher doses than those intended by doctors. The hypothesis was that a patient at risk of developing NUD would employ multiple strategies to achieve a higher MEDD, resulting in a discrepancy between the MEDD intended by the doctors and the overlapping MEDD that the patient achieves through these strategies. Consequently, the MEDD ratio is defined as follows (Fig. 1A):
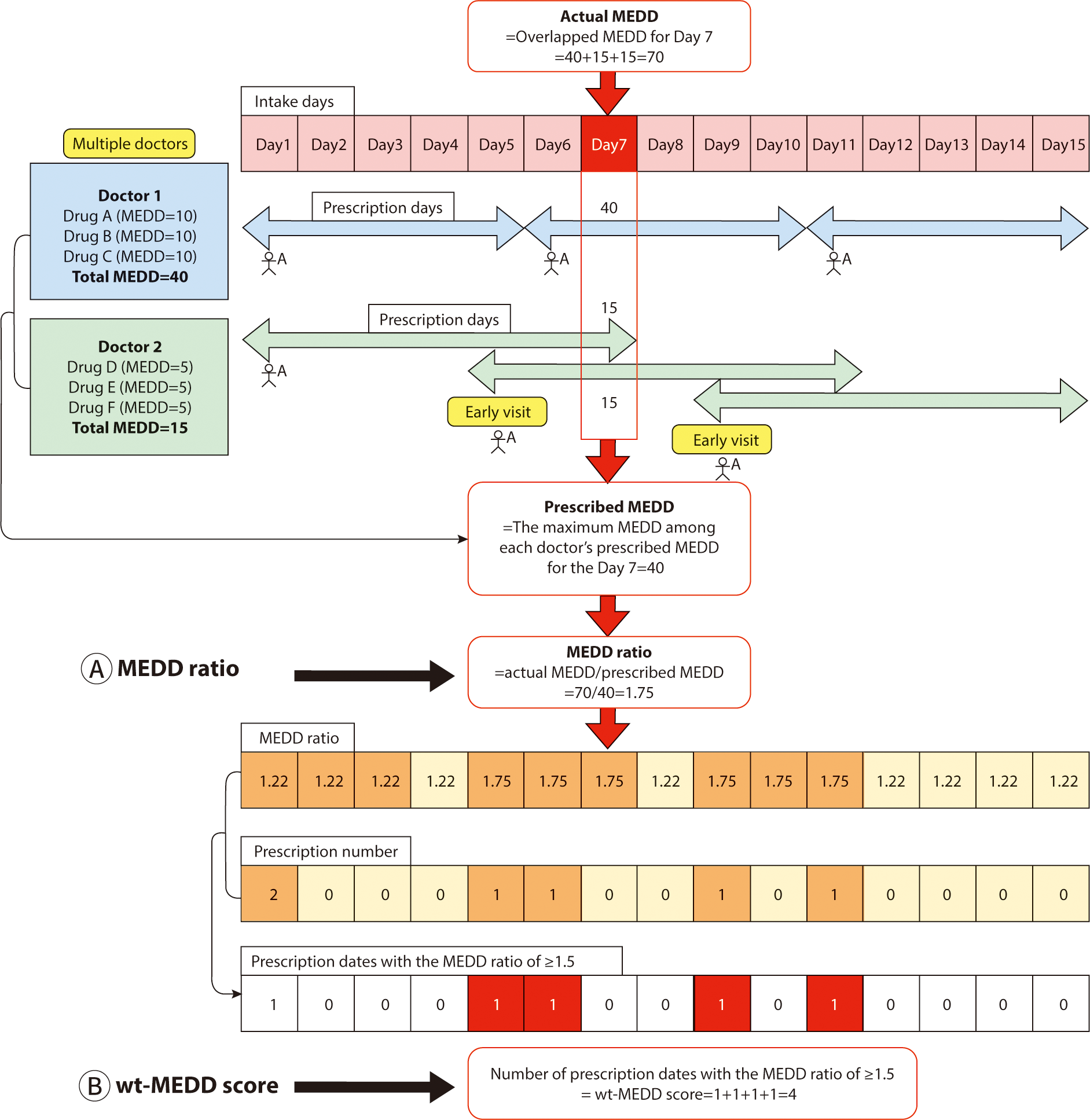
For example, doctor 1 prescribes 40 MEDD to patient A, deeming it an appropriate dosage. However, patient A subsequently visits Doctor 2 to obtain an additional prescription for narcotics. Unaware of the previous prescription from Doctor 1, Doctor 2 prescribes an additional 15 MEDD, considering it suitable for patient A. Later, patient A returns to doctor 2 before the scheduled follow-up, claiming to have lost the previous prescription, and receives another 15 MEDD. Consequently, patient A ends up receiving a total of 70 MEDD of narcotics, which is 1.75 times the highest intended dose of 40 MEDD prescribed by the doctors. The MEDD ratio, defined as the ratio between the actual MEDD received and the maximum intended MEDD prescribed by the doctors, is thus 1.75 in this scenario (Fig. 1A).
We defined the wt-MEDD score as follows (Fig. 1B):
In Fig. 1, patient A consults doctor 1 on days 1, 6, and 11, and sees doctor 2 on days 1, 5, and 9. This results in a total of five prescription dates for patient A (days 1, 5, 6, 9, and 11). Out of these, the number of dates where the MEDD ratio is ≥1.5 amounts to four (day 5, 6, 9, and 11). The wt-MEDD score, which is defined as the number of prescription dates with a MEDD ratio of ≥1.5, is therefore 4, as shown in Fig. 1B. If patient A persists in obtaining narcotics prescriptions from multiple doctors and visiting them earlier than scheduled, the wt-MEDD score will continue increasing.
The choice of a MEDD ratio of 1.5—higher than 1.0 but lower than 2.0—was made to accommodate minor discrepancies between the intended MEDD prescription by doctors and the actual MEDD, such as during initial dose adjustments at the start of narcotic prescriptions. This range also effectively identifies abnormal prescriptions that require further review. Ratios below 1.5 may be overly sensitive, failing to distinguish significant deviations between the actual and intended MEDD. Institutions can adjust the cut-off MEDD ratio based on their preferences, opting for less than 1.5 to increase sensitivity or more than 1.5 to increased specificity. The wt-MEDD score served as a proxy for NUD because repeated prescriptions with a high MEDD rate suggest that the patient is consistently receiving more narcotics than originally prescribed by the physician.
We investigated the clinical applicability of the wt-MEDD score, specifically to monitor abnormal narcotics prescription patterns in a hospital setting. It is necessary to identify both the doctors and patients involved in these practices and to provide them with feedback. To this end, we utilized the wt-MEDD score to compile lists of doctors and patients associated with abnormal prescription patterns. We identified doctors with outlier wt-MEDD scores and similarly generated a list of patients exhibiting outlier scores to closely monitor their prescription behaviors. These individuals were characterized by a two-tailed P-value of <0.001, corresponding to a Z score of ≥3.29 or ≤–3.29. We then extracted the lists of doctors and patients with these outlier wt-MEDD scores and determined the cut-off score for both groups to effectively monitor and address abnormal narcotics prescribing patterns.
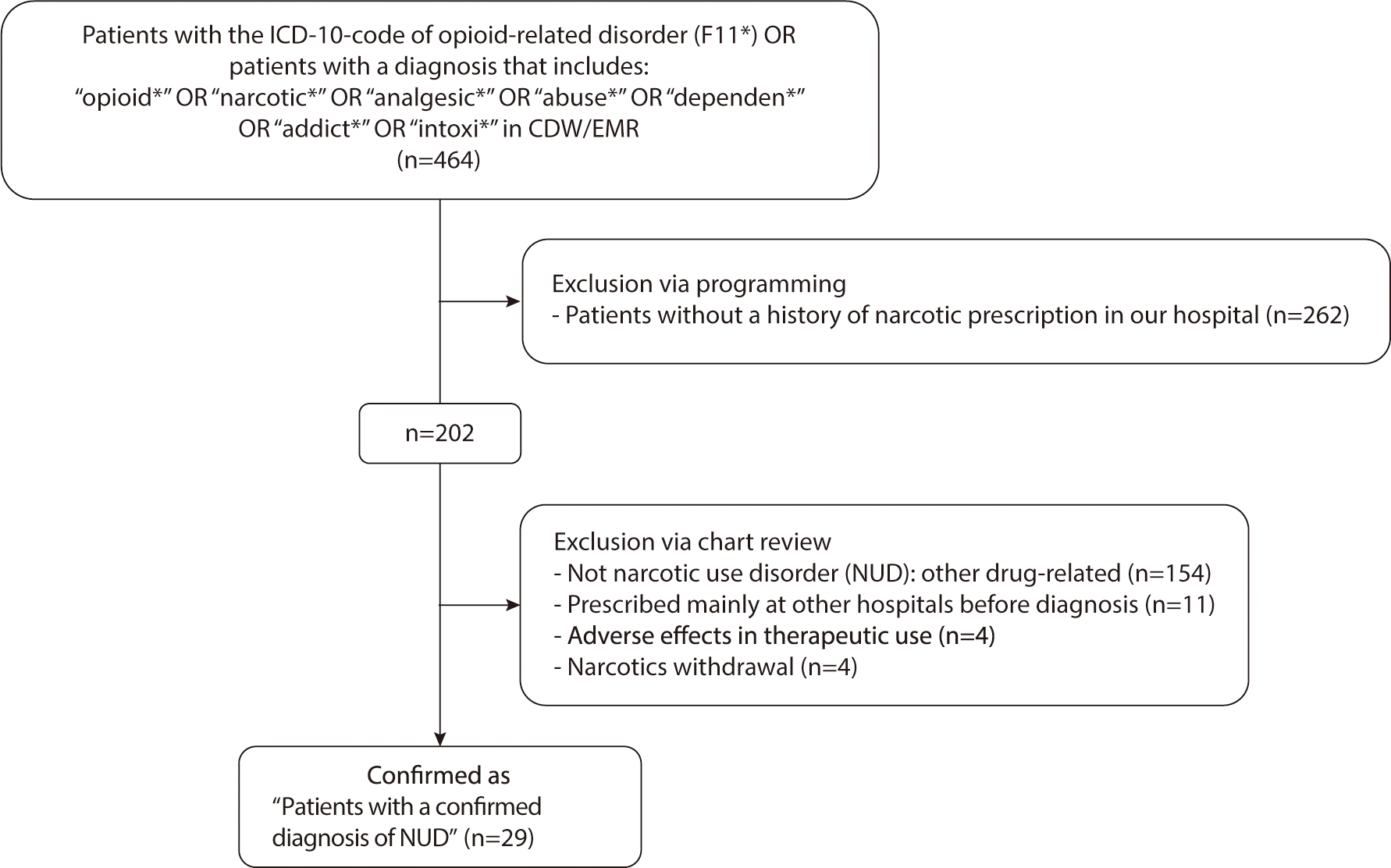
Second, we examined whether the wt-MEDD score could be utilized to identify patients with NUD at an earlier stage. Our analysis focused on determining the optimal cut-off value of the wt-MEDD score for detecting patients diagnosed with NUD by physicians, aiming for the highest sensitivity and specificity. If the cut-off value demonstrated high sensitivity and specificity, and if it was reached before the doctors' diagnosis of NUD, it could serve as an early indicator for NUD detection. A list of patients diagnosed with NUD by doctors was extracted from the clinical data warehouse using the codes from the 10th revision of the International Statistical Classification of Disease and Related Health Problems (ICD-10) and diagnostic terms in the doctors’ medical chart. The accuracy of the NUD diagnoses was verified by ensuring that the chart records met the diagnostic criteria outlined in the DSM-IV-TR or DSM-V. We selected only those patients who had been repeatedly prescribed narcotics at our hospital prior to their NUD diagnosis. Patients diagnosed with NUD at other hospitals, who had little or no history of narcotics prescriptions at our facility, were excluded. Two physicians reviewed the electronic medical record charts to confirm the accuracy of the diagnoses (Fig. 2). Any prescriptions issued to these patients after their NUD diagnosis were omitted from the analysis.
We also analyzed the optimal cut-off values, sensitivity, and specificity of other high-risk NUD indexes (such as the PDMP monitoring categories) and their combinations to confirm the effectiveness of the wt-MEDD score. To determine whether the differences in sensitivity and specificity between the wt-MEDD score and other indexes were statistically significant, the McNemar test was performed.
We observed the time points at which the cut-off values of the wt-MEDD score and other NUD high-risk indexes were reached, as well as the time points at which NUD was diagnosed by a doctor in a patient case. This investigation aimed to determine whether the wt-MEDD score cut-off value could be used to identify NUD earlier. The paired t-test was employed to compare the mean time from the first prescription of narcotics to the point of reaching the wt-MEDD score cut-off value and the subsequent NUD diagnosis by doctors.
We analyzed the narcotic prescriptions of 221,887 patients, which were prescribed by 8,737 doctors from July 2000 to June 2018.
Considering the definition of narcotic abuse and the conditions of PDMP monitoring, the wt-MEDD score and NUD high risk indexes (MEDD, prescription days, prescribing frequency and duration, number of prescribing doctors, and number of early receipt of narcotics before the scheduled visit) were selected for analysis.
A clinical data warehouse is a near-real-time database that consolidates data from various clinical sources. A web-based browser facilitated the extraction of a list of patients who met the inclusion criteria, along with their corresponding electronic medical records. Information regarding the prescribed patients was downloaded from the clinical data warehouse into five tables: basic information, narcotic prescription, admission, surgery, and diagnosis records.
We calculated the activity of each narcotic based on its mode of administration—tablet, patch, or injection. The table included information on the MME conversion factor, derived from PDMP supplements [15]. The MEDD is calculated by multiplying the MME conversion factor by the daily dose. When MME conversion factor information for a specific drug was not available, we estimated it from the relevant literature [16].
Among the five types of downloaded tables, the narcotic prescription table included information such as the name of the prescribed drug, the date of prescription, the number of days prescribed, and the MEDD (Supplement 1). To calculate the overlapping MEDD for a specific intake date, a new table was created. This table transformed each intake date for a patient into individual rows—not just the prescription dates—by reformatting the data from the prescription table (Supplement 2).
A 3-month interval was established as the measurement period for the time-series analysis, specifically January-March, April-June, July-September, and October-December. The total number of prescriptions issued during each 3-month period was calculated. To analyze temporal changes in MEDD per patient, the highest MEDD recorded in each 3-month interval was identified and compared with the highest MEDDs from the other intervals.
Sample size estimation was not performed because this study included all target participants.
R (R Foundation for Statistical Computing, Vienna, Austria; URL: http://www.R-project.org/, ver. 3.6.0) and RStudio (RStudio, Boston, MA, USA; URL: http://www.rstudio.com/, ver. 1.2.1335) were used for statistical analyses. The ‘dplyr’ package in R was used to analyze data, and the ‘ggplot2’ package was used to generate graphs. Outlier analysis was performed using the ‘outliers’ package. Cut-off value, sensitivity, specificity, and accuracy were calculated using the ‘pROC’ package. A two-tailed P-value of less than 0.05 was considered statistically significant.
Results
This study included 221,887 patients who received prescriptions for narcotics, written by 8,737 doctors, totaling 555,097 narcotic prescriptions. Upon reviewing the records of 464 patients diagnosed with NUD in the CDM, only 29 were confirmed to have NUD following repeated narcotic prescriptions at our hospital. The majority of patients were diagnosed with NUD at other hospitals before being transferred to our facility (Fig. 2).
The cut-off wt-MEDD score for patient outliers (n=996) was 10.5 (P<0.001). The list of doctor outliers (n=23) for the wt-MEDD score can be found in Supplement 3 and Fig. 3. This list, which included both doctors and patients, was extracted and provided to the hospital committee to monitor prescription abnormalities and offer feedback to the involved doctors.
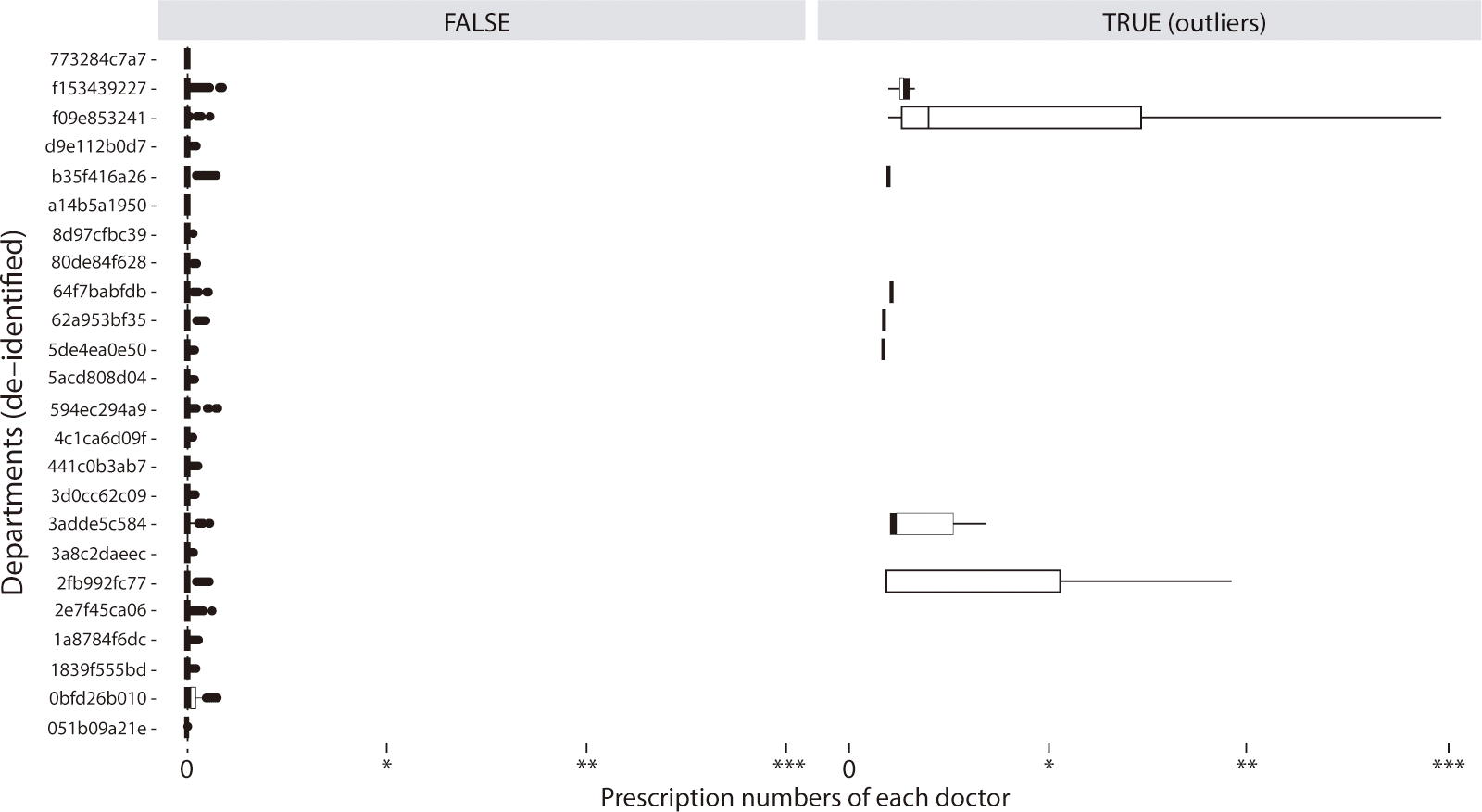
Table 1 compares the wt-MEDD scoring system with NUD high-risk indexes for detecting diagnosed NUD. The optimal cut-off value for the wt-MEDD score, which demonstrated the highest sensitivity and specificity, was ≥10.5. This value was similar to the cut-off for outlier wt-MEDD scores (P<0.001). The median wt-MEDD score among the 29 patients diagnosed with NUD was 52 (25th–75th quartiles=25–115), indicating that most patients with NUD had multiple prescriptions with a high MEDD ratio, exceeding the intended prescription level. When compared to other NUD high-risk indexes, the wt-MEDD score exhibited the highest sensitivity and specificity (100.0% and 99.6%, respectively; Table 1). The McNemar test revealed that the sensitivity and specificity of the wt-MEDD score were significantly superior to those of other indexes (P<0.001), with the exception of two indexes: the quarterly number with a doctor number of ≥4 and the number of prescriptions ≥10 days earlier than the scheduled visit (Table 1).
To improve the ability to detect NUD, we combined the NUD high-risk indexes with the wt-MEDD score and assessed their sensitivity and specificity. A combined model that included the highest overlapping MEDD, total number of prescriptions, and total number of doctors (triple-test) demonstrated excellent sensitivity and specificity, at 96.6% and 99.5% respectively. When the wt-MEDD score was used in conjunction with the triple-test, the sensitivity and specificity further improved to 96.6% and 99.7%, respectively. These results suggest that the wt-MEDD score, when combined with other NUD high-risk indexes, is effective for screening patients with NUD (Table 1).
In all 29 patients diagnosed with NUD, the time point at which the wt-MEDD cut-off score was reached occurred earlier than the time point of the doctor's initial diagnosis of NUD. The average time to reach the wt-MEDD cut-off score was 1,024 days (median [quartile 1–quartile 3], 361 [192–2,323]) from the first narcotics prescription. In contrast, the average time until NUD diagnosis was 2,578 days (median [quartile 1–quartile 3], 2,342 [1,396–4,030]). This resulted in an average difference of 1,554 days (95% CI, 1,096–2,010 days, paired t-test, P<0.001).
To provide an example of the clinical application of the new methodology (wt-MEDD score) in identifying cases of NUD, we selected a patient diagnosed with NUD who employed multiple strategies to obtain a higher number of narcotics prescriptions. We retrospectively observed the time-sequential changes in the wt-MEDD score and the NUD high-risk indexes for this patient up until the NUD diagnosis was made (Fig. 4). Fig. 4A demonstrates a gradual increase in the patient's wt-MEDD score over time. Initially, the score was 10 in November 2011, and the patient received a diagnosis of NUD in October 2017. If the wt-MEDD score had been utilized as a screening tool for this patient, it could have potentially led to a diagnosis of NUD 6 years earlier.
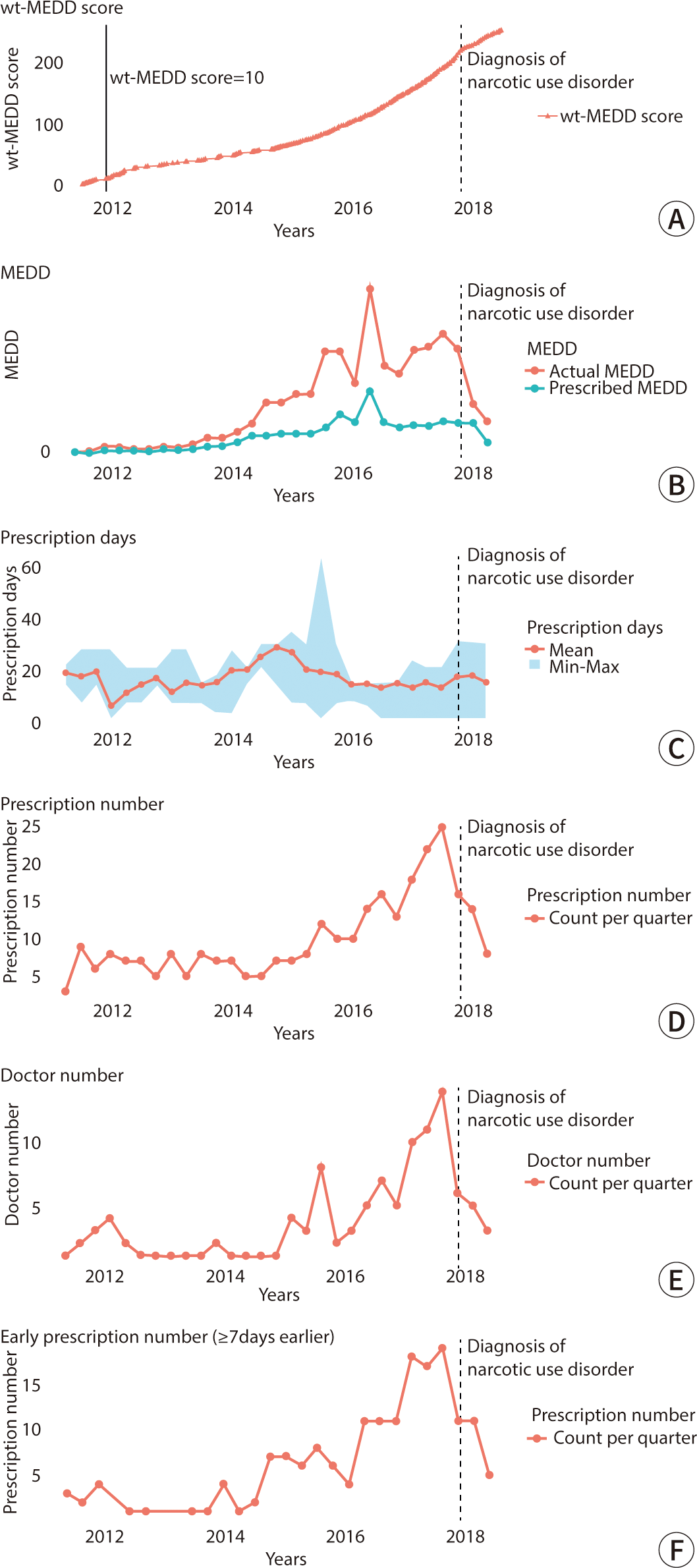
The difference between the actual MEDD and the intended MEDD (MEDD ratio) continued to increase until the diagnosis of NUD was made (Fig. 4B). Concurrently, the number of prescription days (mean, minimum, and maximum) showed an increase just prior to the rise in the MEDD ratio (Fig. 4C). Similarly, the patterns of increase in the number of prescriptions (Fig. 4D), the number of prescribing doctors per 3-month period (Fig. 4E), and the instances of early receipt of narcotics for more than 7 days (Fig. 4F) mirrored the trend observed in the MEDD ratio. These findings suggest that employing multiple strategies can elevate the MEDD ratio, potentially leading to a diagnosis of NUD. Despite the use of various strategies to obtain more narcotics, it is possible to effectively screen for NUD at an early prescription stage using only the wt-MEDD score, without the need for multiple indicators.
Discussion
The wt-MEDD score demonstrated remarkable effectiveness in identifying early patterns of narcotic prescriptions in patients who were later diagnosed with NUD. This score reflects the number of prescription dates with a high MEDD ratio. A wt-MEDD score greater than 10.5 marked patients as significant outliers, aligning with the optimal cut-off value used by physicians to detect NUD. With a sensitivity of 100% and a specificity of 99.6%, a wt-MEDD score of 10.5 proved highly effective in identifying patients diagnosed with NUD. These results indicate that a wt-MEDD score of 10.5 could serve as a screening tool to detect patients with NUD, particularly those engaging in behaviors like "doctor shopping" to obtain excessive amounts of narcotics.
Our hospital undertook an analysis of abnormal patterns in narcotic prescriptions to prevent NUD in patients by providing feedback to prescribing doctors. Initially, we consulted the CDC guideline [7], which proved both reasonable and useful, as evidenced by its alignment with the optimal cut-off values identified in our study for detecting patients with NUD. However, adherence to the CDC guideline alone was insufficient for categorizing a patient with NUD. To provide prescribing doctors with clearer information reflective of NUD, we took into account scenarios that could be problematic without exception. For instance, a patient receiving high doses of narcotics inconsistent with the prescribing doctor's intentions was identified as a problematic situation and thus was considered appropriate for defining NUD.
In the United States, the PDMP system automatically calculates the MEDD for prescribed narcotics. It provides the total MEDD for multiple prescriptions on the date they are issued; however, it does not calculate the overlapping MEDD that results from multiple prescriptions on a specific intake date [17]. Although the Ohio Automated Rx Reporting System displays daily MME for prescribers, this information is not stored, making it challenging to reconstruct later [17]. Previous studies have examined overlapping prescriptions, focusing either on concurrent benzodiazepine and narcotic prescriptions [18] or on overlapping prescriptions without considering the overlapping MEDD [19]. To our knowledge, this study is the first to evaluate the wt-MEDD score, which is based on the number of days with a high MEDD ratio, as a tool for identifying abnormal prescription patterns.
The wt-MEDD score is effective in identifying abnormal prescriptions, irrespective of the methods patients employ to obtain more narcotics. Additionally, a graph depicting the MEDD ratio over time can be made available for each patient in the outpatient clinic. The capability to quickly observe temporal changes through the graph can significantly reduce the time required to detect unusual prescription patterns.
An opioid-risk tool, previously reported and based on fixed patient characteristics such as a history of alcoholism, has been shown to have a primary prevention effect on NUD [20]. However, it does not contribute to secondary prevention. In contrast, monitoring the wt-MEDD score facilitates screening prior to an increase in the number of abnormal prescriptions.
Calculating the wt-MEDD score based on overlapping MEDD could lead to an increase in data volume, as each intake date—rather than just the prescription date—adds an additional row to the table. In our study, the data size for tables based on each intake date was five times larger than those based on each prescription date. However, limiting the analysis to patients who have been prescribed narcotics, rather than including all hospital patients, could alleviate the burden of data analysis. Additionally, our study only included a small number of patients diagnosed with NUD, possibly due to physicians' reluctance to diagnose NUD. This issue is particularly acute for patients who have received narcotics prescriptions from more than one department, where cross-departmental liability issues may complicate the diagnosis of NUD. Consequently, there could be a larger number of undiagnosed NUD cases, especially among those categorized as wt-MEDD outliers. Moreover, our analysis did not include narcotic prescriptions for cancer patients, inpatients, or those receiving relatively low doses of narcotics such as codeine and tramadol. These groups are expected to be analyzed using different criteria. This study was retrospective, suggesting that a prospective trial might be necessary to determine if monitoring the wt-MEDD score can reduce the incidence of NUD. Additionally, since our study population was limited to a single hospital, it did not account for narcotics prescribed to patients at other facilities. Given that patients with NUD are likely to receive narcotics prescriptions from multiple hospitals, an integrated system to monitor narcotic prescriptions nationwide becomes essential. In the United States, for example, the PDMP manages all narcotic prescriptions at the state level [21]. However, the variation in narcotic policies between different hospitals and countries could complicate such analysis [22]. Nonetheless, if the system can effectively detect NUD through hospital prescriptions, a method for confirming the wt-MEDD score might prove universally beneficial.
We defined the wt-MEDD score as the number of prescription days with a high MEDD ratio, based on the definition of narcotic abuse. The wt-MEDD score identified patients diagnosed with NUD with greater sensitivity and specificity than other metrics. Therefore, monitoring the wt-MEDD score could enable early interventions for irregular narcotics prescription patterns by doctors and help prevent the development of NUD in patients.

