Introduction
In Korea, cancer remains a major cause of death, with liver cancer being the second most common cause of cancer-related deaths following lung cancer in 2020 (lung cancer: 36.4 deaths, liver cancer: 20.6 deaths per 100,000, according to statistics from the National Statistical Office) [1]. Furthermore, liver cancer represents the most substantial economic burden, particularly impacting individuals in their most productive years, underscoring its critical importance as a health issue in the country [2]. Thus, this paper aims to examine the latest treatment trends for advanced-stage hepatocellular carcinoma (HCC), referencing the newly revised 2022 HCC guidelines and the 2022 Barcelona Clinical Liver Cancer (BCLC) system [3].
HCC often develops in the context of chronic hepatitis, primarily driven by innate immune activation. However, certain causes are associated with specific immune dysfunctions. Chronic viral hepatitis can lead to liver cancer by initiating inflammatory innate immune responses and fostering an abnormal adaptive immune reaction that fails to eliminate the hepatitis virus [4]. Approximately half of HCC patients receive systemic therapy. Traditionally, sorafenib or lenvatinib were used as first-line treatments, followed by regorafenib, cabozantinib, or ramucirumab as second-line options. However, since 2020, immune checkpoint inhibitors (ICIs) have shown significantly improved overall survival rates compared to sorafenib. Therefore, the combination therapies of atezolizumab with bevacizumab and durvalumab with tremelimumab have become the preferred first-line treatments.
This review aims to provide an overview of recent advancements in treating advanced HCC, based on the updated 2022 HCC guidelines and the BCLC system. It specifically updates the following topics: first-line systemic therapy, second-line systemic therapy following the failure of first-line treatment, and the side effects of systemic chemotherapy agents.
Ethics statement
As this study is a literature review, it did not require institutional review board approval or individual consent.
First-line systemic therapy
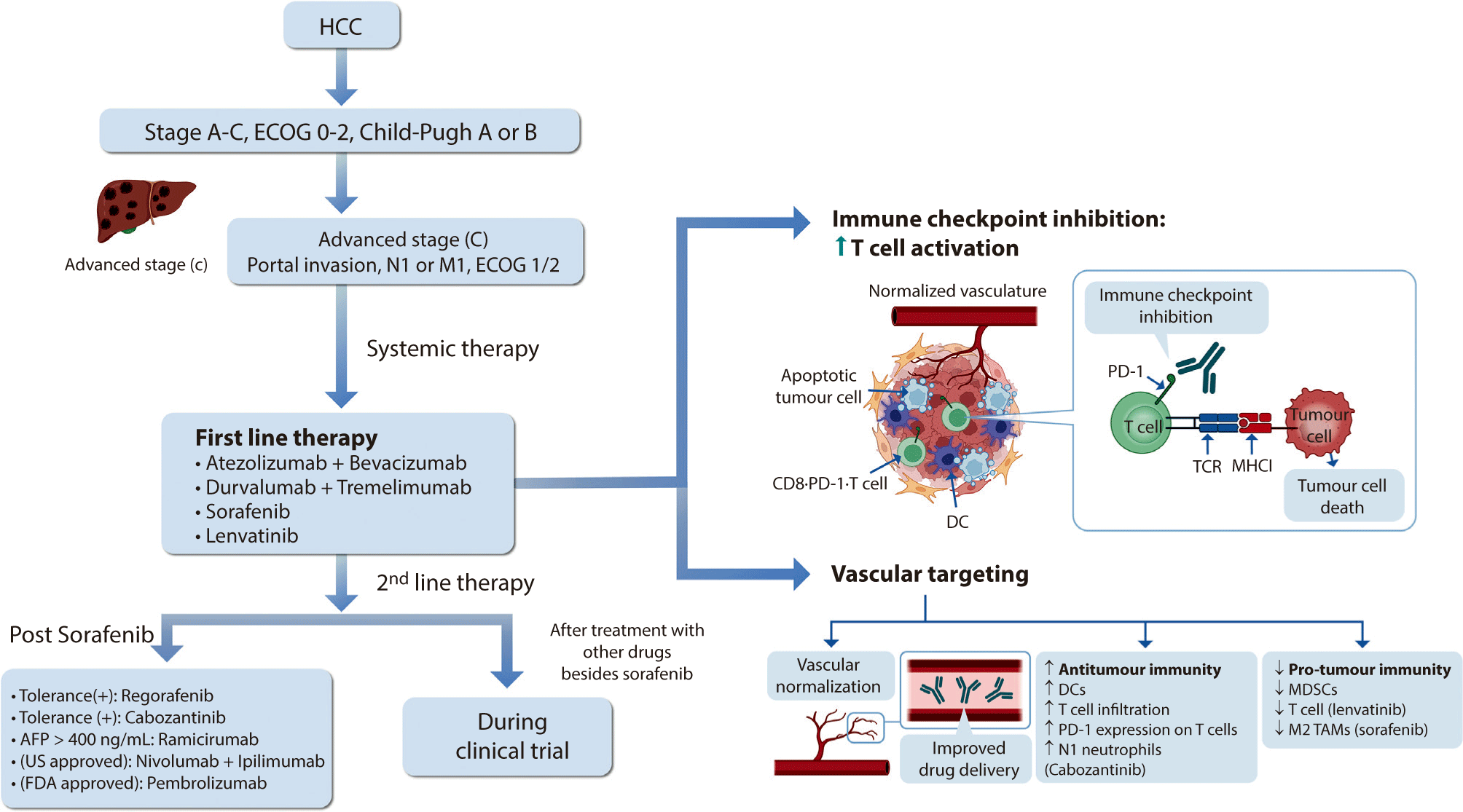
The first-line treatments for advanced HCC are discussed sequentially, starting with the most preferred medications. Fig. 1 provides a visual overview intended to facilitate a deeper understanding of the comprehensive treatment strategy for advanced HCC.
Atezolizumab is an intravenous IgG1 monoclonal antibody that targets programmed death ligand 1 (PD-L1) on the surface of cancer cells, preventing its interaction with the receptor. Bevacizumab, another intravenous IgG monoclonal antibody, binds to vascular endothelial growth factor (VEGF), thereby inhibiting angiogenesis and tumor growth.
In the IMbrave150 study, patients with advanced HCC who were treated with a combination of atezolizumab and bevacizumab exhibited a significant increase in median overall survival and progression-free survival (PFS) compared to those treated with sorafenib (6.8 months; 95% CI, 5.7–8.3 vs. 4.3 months; 95% CI, 0.47–0.76, P<0.001). This marked improvement prompted the Food and Drug Administration (FDA) to approve the combination therapy in 2020. The study excluded patients with autoimmune diseases, concurrent HBV and HCV infections, or untreated esophageal or gastric varices. The primary causes of HCC were chronic infections, with HBV and HCV accounting for 49% and 21% of cases, respectively. All participants had preserved liver function (Child-Pugh class A), and 26% had low-risk varices at baseline, with 11% undergoing treatment. The study was halted at the first interim analysis after demonstrating significant improvements in both overall survival and PFS [5]. Recent updates indicate that after a median follow-up of 15.6 months, the combination of atezolizumab and bevacizumab achieved a median overall survival of 19.2 months, the longest recorded in any phase 3 trial for advanced HCC. Additionally, the overall response rate reached 30%, more than double that of sorafenib [6]. In 2023, a systematic literature review and network meta-analysis were conducted to indirectly compare the combination of atezolizumab and bevacizumab with other treatments for unresectable HCC. The analysis showed that this combination therapy leads to improved overall survival, supporting its use as a first-line treatment for patients with unresectable liver cancer. However, it is important to recognize that this combination may not be suitable for all patients, and careful evaluation is necessary to determine the most appropriate treatment for each individual [7–10].
Tremelimumab is an intravenous IgG2 monoclonal antibody that targets CTLA-4 on activated T-cells, thereby blocking its interaction with the ligands CD80 and CD86. Durvalumab, a fully human IgG1 antibody, binds to PD-L1, inhibiting its interaction with PD-1 and reversing peripheral tolerance against tumor cells [11,12].
On October 15, 2021, the HIMALAYA study demonstrated that combination therapy significantly improved survival compared to sorafenib, with survival times of 16.43 months versus 13.77 months, respectively (hazard ratio [HR], 0.78; 96.02% CI, 0.65–0.92, P=0.0035). This finding met the primary endpoint of the study. The trial enrolled patients with BCLC stage B or C, Child-Pugh class A, ECOG PS 0 or 1, and at least one measurable lesion according to RECIST 1.1 criteria. Patients requiring non-drug treatment for ascites, those with major portal vein thrombosis, or those co-infected with HBV and HCV were excluded from the study. Patients requiring non-drug treatment for ascites, those with major portal vein thrombosis, or those co-infected with HBV and HCV were excluded. The dosing regimen was determined based on pharmacokinetic studies. The STRIDE regimen, which involves administering 300 mg of tremelimumab once, followed by 1,500 mg of durvalumab in the first cycle and then 1,500 mg of durvalumab alone every 4 weeks, proved effective. It was noted that a high dose of tremelimumab could enhance CD8+ T cell levels in peripheral blood, potentially boosting the efficacy of the combination therapy. This study led to the FDA approval of the combination therapy in October 2022, and it received approval from the European Medicines Agency in January 2023 for the treatment of unresectable HCC. An update in January 2023 from the HIMALAYA study indicated that the median follow-up periods for STRIDE, durvalumab, and sorafenib were 49.12 months (95% CI, 46.95–50.17 months), 48.46 months (95% CI, 46.82–49.81 months), and 47.31 months (95% CI, 45.08–49.15 months), respectively [12–14].
Sorafenib is an oral multi-tyrosine kinase inhibitor (TKIs) that targets various receptors, including VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-β, Raf-1, and c-kit. Its anticancer effects are derived from the dual inhibition of angiogenesis and tumor cell proliferation. Additionally, sorafenib inhibits the phosphorylation of the initiation factor eIF4E and promotes cancer cell death by reducing the levels of the anti-apoptotic protein Mcl-1.
The landmark phase 3 SHARP trial enrolled 602 HCC patients, with 97% classified as Child-Pugh class A. This study involved a comparison between a placebo group of 303 patients and a sorafenib group of 299 patients. Among these, 70% had advanced HCC, with underlying conditions including HBV infection (18%), HCV infection (28%), and alcohol-related diseases (26%). Sorafenib was administered orally at a dosage of 400 mg twice daily and significantly increased the median overall survival to 10.7 months, compared to 7.9 months for the placebo group (P<0.001). Therefore, in 2007, sorafenib was approved by the FDA as the first treatment for HCC [15–18].
Lenvatinib, administered orally at a dosage of 12 mg/day for individuals weighing over 60 kg and 8 mg/day for those under 60 kg, is a molecular targeted therapy. It targets multiple receptors, including VEGFR-1/2/3, FGFR-1/2/3/4, PDGFR-α, RET, and c-kit. This therapy inhibits angiogenesis and disrupts fibroblast growth factor signaling in human HCC models [19]. In the multinational phase 3 REFLECT study, lenvatinib demonstrated non-inferiority to sorafenib in terms of survival, with survival times of 13.6 months versus 12.3 months (HR 0.92, 95% CI, 0.79–1.06). Consequently, in 2018, lenvatinib was approved as the first-line systemic treatment for HCC, representing the first such approval in a decade since sorafenib [20,21].
Although immunotherapy is highly effective, its use is limited in patients with recurrent HCC after liver transplantation due to the high risk of allograft rejection. However, retrospective studies have indicated that sorafenib and lenvatinib are safe for these patients. In a retrospective cohort study of 45 patients with recurrent HCC post-liver transplantation treated with lenvatinib, the median overall survival was 14.5 months (95% CI, 0.8–28.2), with a median PFS of 7.6 months (95% CI, 5.3–9.8) and an objective response rate of 20% [22]. This suggests that lenvatinib is a valuable first-line treatment option for advanced HCC, especially in patients concerned about resistance, those unable to undergo timely upper gastrointestinal endoscopy, or for whom immunotherapy is contraindicated. Lenvatinib was also evaluated in a multinational, multicenter trial assessing the clinical outcomes of multiple kinase inhibitors in cancer patients whose disease had progressed following combination therapy with atezolizumab and bevacizumab. The study included patients classified as Child-Pugh class A and BCLC stage B or C. The results showed that patients treated with lenvatinib experienced a longer median PFS of 6.1 months (95% CI, 1.6–10.5) compared to those treated with sorafenib, which was 2.5 months (95% CI, 1.3–3.8, P=0.004). However, overall survival was similar between the two groups (median overall survival, 16.6 months [95% CI, 3.6–29.6] vs. 11.2 months [95% CI, 2.7–19.6]; P=0.347). Lenvatinib has shown promising efficacy and tolerable safety as a second-line treatment following atezolizumab-bevacizumab therapy. Therefore, recent guidelines from NCCN, ASCO, ESMO, EASL, and the KLCA-NCC in Korea recommend considering lenvatinib as a second-line option after atezolizumab-bevacizumab treatment. Furthermore, transarterial chemoembolization, the standard treatment for BCLC-B liver cancer, has been shown to be effective in extending PFS and overall survival when used sequentially or in combination with lenvatinib, as demonstrated in studies conducted in China and Japan. However, additional prospective research, including studies with Western populations, is necessary to confirm these benefits. Finally, the combination therapy of lenvatinib with ICIs has been explored across various cancer types and has received FDA approval for use in advanced renal cell carcinoma. The combination of lenvatinib with pembrolizumab is particularly noteworthy, as lenvatinib suppresses angiogenesis and immune inhibition in the tumor microenvironment, enhancing the anti-tumor immune response of pembrolizumab through a synergistic effect. In this context, the phase 3 LEAP-002 study, which evaluates the effectiveness of lenvatinib combined with pembrolizumab versus lenvatinib monotherapy in advanced HCC, has been conducted. Although there were numerical improvements in PFS and overall survival, the combination therapy did not reach statistical significance in enhancing overall survival and PFS compared to placebo. Nonetheless, this study is significant as it suggests that combining lenvatinib with immunotherapy could be a viable strategy for treating advanced HCC [23].
Second-line systemic therapy after failure of first-line systemic therapy
No studies have directly compared the effectiveness of various second-line systemic therapies following the failure of sorafenib. However, regorafenib and cabozantinib have shown improved overall survival compared to placebo in such scenarios. Additionally, ramucirumab has been found to enhance overall survival in patients with serum alpha-fetoprotein (AFP) levels above 400 ng/mL, irrespective of resistance to sorafenib. Table 1 summarizes the survival rates and target agents for each drug, organized according to the sequential treatment strategy for advanced HCC. Currently, there is a significant gap in research concerning the use of approved first-line treatments, such as lenvatinib and the atezolizumab/bevacizumab combination, as second-line options following treatment failure. Furthermore, no research results are available on the efficacy of using durvalumab and tremelimumab combination therapy as a second-line systemic treatment after the failure of first-line treatments [24].
Patients who exhibit sorafenib resistance may consider regorafenib as a second-line treatment. Regorafenib operates through three primary mechanisms: angiogenesis inhibition, cell proliferation control, and tumor microenvironment regulation. This oral multi-kinase inhibitor targets a variety of receptors, including VEGFR 1-3, TIE-2, PDGFR-β, c-KIT, RET, RAF-1, and BRAF. The simultaneous blockade of VEGF and TIE-2 receptors is thought to significantly improve the constriction of tumor blood vessels. Studies indicate that regorafenib also possesses immune-modulating properties; it helps prevent immune suppression, regulates macrophages, and enhances the proliferation and activation of CD8+ T cells, thereby boosting the anti-tumor immune response.
The RESORCE study demonstrated that patients treated with regorafenib experienced a significantly longer median overall survival compared to the control group (10.6 months vs. 7.8 months; HR 0.63; 95% CI, 0.50–0.79; P<0.001). This finding established regorafenib as the first second-line systemic therapy to show a survival benefit, culminating in its FDA approval in April 2017 for use as a second-line treatment [25–28].
This oral molecular targeted therapy simultaneously inhibits MET, VEGFRs, RET, and KIT, making it effective even in cases resistant to sorafenib. It functions by inhibiting the activity of multiple tyrosine kinases and preventing receptor phosphorylation, thereby halting signal transduction. This mechanism results in the death of cancer cells, decreased proliferation, inhibition of metastasis, reduced tumor blood vessel formation, and ultimately, tumor shrinkage.
A similar result was observed in the multinational phase 3 CELESTIAL trial, where cabozantinib significantly extended median overall survival compared to the placebo group (10.2 months vs. 8.0 months; HR, 0.76; 95% CI, 0.63–0.92; P=0.005). Therefore, cabozantinib received approval from the EMA and FDA for the treatment of HCC patients who had previously been treated with sorafenib, recommending a daily dose of 60 mg. In November 2021, findings from the COSMIC-132 trial, which investigated the combination of atezolizumab and cabozantinib, were published. The study showed a significant improvement in PFS, with an HR of 0.63. However, interim data did not show a significant improvement in overall survival compared to sorafenib, pending the final analysis [29]. Similarly, the final results of the COSMIC-312 study, reported in 2024, indicated that although the combination of atezolizumab and cabozantinib continued to show a significant benefit in PFS, it did not enhance overall survival compared to sorafenib. In 2020, a matching-adjusted indirect comparison was performed to indirectly compare the outcomes of the CELESTIAL and RESORCE trials in patients who had received sorafenib as first-line therapy. This comparison assessed the efficacy and safety profiles of cabozantinib and regorafenib. The findings demonstrated that cabozantinib, compared to regorafenib, achieved a similar overall survival and a longer PFS in patients with advanced HCC whose disease had progressed following sorafenib treatment [30–32].
Ramucirumab is an intravenous monoclonal antibody that specifically targets VEGFR-2. Unlike bevacizumab, ramucirumab exhibits a broader inhibitory profile by blocking all forms of VEGF from binding to VEGFR-2, thereby effectively halting angiogenesis due to its high binding affinity. Elevated AFP levels are typically associated with poor prognosis and increased angiogenesis, as well as heightened VEGFR expression. In the REACH-2 trial, which included patients with serum AFP levels of 400 ng/mL or higher, BCLC-B/C, ECOG PS 0/1, and Child-Pugh class A, participants who received 8 mg/kg of ramucirumab biweekly demonstrated a significant improvement in overall survival compared to those in the placebo group (8.5 months vs. 7.3 months; HR, 0.71; 95% CI, 0.531–0.949; P=0.0199) [33–35].
Nivolumab is an intravenous PD-1 inhibitor and a recombinant human IgG4 monoclonal antibody. It functions by binding to the PD-1 receptor on T cells' surfaces, thereby restoring their ability to attack cancer cells. Ipilimumab targets the CTLA-4 receptor on the cell membrane, blocking its interaction with the ligands CD80 and CD86. This combination is conditionally approved by the FDA as a second-line treatment following sorafenib. The CheckMate 040 trial assessed the efficacy and safety of nivolumab and ipilimumab in patients with advanced HCC who had previously received sorafenib treatment. The study demonstrated that the combination therapy led to significant and durable responses, resulting in the approval of the regimen in the United States. This regimen involves administering nivolumab at 1 mg/kg and ipilimumab at 3 mg/kg every 3 weeks for four doses, followed by nivolumab at 240 mg every two weeks. However, this combination has not yet been incorporated into domestic or BCLC guidelines [36–38]. In 2024, the 5-year results from this cohort were published, confirming the initial findings. The combination therapy in arm A showed clinically meaningful responses and extended survival benefits for patients with advanced HCC previously treated with sorafenib, further endorsing its use as a second-line treatment [39,40].
Pembrolizumab is a human IgG4 monoclonal antibody that targets the PD-1 receptor, inhibiting its interaction with PD-L1 and PD-L2. Although the KEYNOTE-240 study, which compared pembrolizumab with a placebo, did not achieve statistically significant results in its final analysis, the findings still underscore the drug's antitumor activity as a second-line treatment for HCC. These results have also set the stage for further investigations [41]. In November 2018, pembrolizumab was granted accelerated approval by the FDA, following the outcomes of the global phase 2 KEYNOTE-224 study that included patients with advanced HCC who had previously been treated with sorafenib.
In 2022, the phase 3 KEYNOTE-394 study evaluated pembrolizumab as a second-line treatment compared to a placebo in Asian patients. Pembrolizumab was administered at a dose of 200 mg every three weeks, mirroring the placebo group's regimen. The study results indicated a significant improvement in median overall survival (14.6 vs. 13.0 months; HR for death, 0.79; 95% CI, 0.63–0.99, P=0.0180) and PFS (2.6 vs. 2.3 months; HR for progression or death, 0.74; 95% CI, 0.60–0.92, P=0.0032). Furthermore, the objective response rate was markedly better (12.7% vs. 1.3%, P<0.0001), reinforcing the recommendation to use pembrolizumab as a second-line treatment for this patient group [42,43].
Side effects of systemic chemotherapy agents
Although sorafenib offers significant benefits to patients, it also has side effects similar to those of other TKIs. Sorafenib targets the VEGF receptor pathway and Raf kinase, both of which are essential for maintaining physiological functions and homeostasis in the body. Inhibiting these signaling pathways can result in therapeutic benefits but also potential side effects. Currently, side effects such as hypertension, thyroid dysfunction, hand-foot syndrome, and fatigue are associated with the inhibition of various tyrosine kinases. Additionally, hypertension, arterial thromboembolism, proteinuria, wound complications, bleeding, and gastrointestinal perforation are closely linked to the VEGF pathway. While most side effects are manageable, severe adverse reactions like cardiac shock or hemorrhage can be life-threatening and may lead to the discontinuation of chemotherapy. Therefore, it is crucial to review these side effects thoroughly [44,45].
Hypertension is one of the most commonly reported side effects of angiogenesis inhibitors such as sorafenib and may manifest within 2 weeks of initiating treatment. This hypertension results from the toxicity inherent in the mechanism of action of sorafenib and is sometimes considered a predictive marker of the drug’s antitumor efficacy, suggesting that sorafenib is functioning effectively. Over time, the incidence of hypertension may decrease, indicating that the body might be adapting to the drug, which could potentially reduce the cardiovascular risks observed at the beginning of treatment. Therefore, patients should monitor their blood pressure weekly after starting treatment and can manage hypertension with standard antihypertensive medications without needing to reduce the sorafenib dose. Recent studies suggest that monitoring the steady-state concentration of sorafenib may help in avoiding severe toxicities, including hypertension.
Hypertension induced by TKIs is linked to several complications, including a reduction in left ventricular ejection fraction, heart failure, and coronary artery disease. In a randomized, double-blind clinical study, myocardial ischemia or infarction occurred in 4.9% of patients treated with sorafenib, compared to only 0.4% in the placebo group. This damage is thought to arise from the inhibition of RAF1 and BRAF kinases, which disrupts the ERK kinase cascade and directly suppresses myocardial cell survival. However, cardiac damage caused by sorafenib can generally be managed effectively if the patient's heart function is closely monitored and appropriate treatment is administered.
Sorafenib treatment is associated with arterial thromboembolism across various cancer types. However, research on the high-risk factors for sorafenib-induced arterial thromboembolism remains limited, and consensus on prevention and management strategies has not been established. Due to the risk of bleeding, preventive anticoagulant measures such as aspirin are not universally recommended when used concurrently. Consequently, patients who experience such events should discontinue sorafenib, and those with atherosclerosis should use the drug with caution.
Patients treated with sorafenib have reported a range of bleeding complications, including nosebleeds, hemoptysis, gastrointestinal bleeding, vaginal bleeding, and even cerebral hemorrhage. A meta-analysis of studies on anti-angiogenic therapy in patients with HCC and renal cell carcinoma showed that sorafenib increased the risk of bleeding events of all grades compared to control groups (OR 1.77, 95% CI, 1.04–3.0). [46] The inhibition of the VEGF pathway may disrupt platelet activation, hinder thrombus formation following trauma, and decrease subendothelial matrix deposition, which in turn raises the risk of bleeding. Given the significant concerns of bleeding and arterial thromboembolism during sorafenib treatment, careful monitoring of patients and tailored treatment approaches are crucial.
Hand-foot skin reactions (HFSR) are the most prevalent dose-limiting toxicity associated with sorafenib, often occurring during treatment and frequently necessitating dose adjustments. This adverse effect significantly impacts the quality of life of patients, making its management a critical component of the treatment plan. HFSR is characterized by symptoms such as erythematous, edematous, and painful blisters on the palms and soles. Typically, these reactions develop within 2 to 4 weeks of starting sorafenib, with a median onset time of 18.4 days. Notably, TKI-induced HFSR often presents with hyperkeratotic lesions surrounded by erythema, primarily affecting areas such as joints, palms, and soles. Meta-analyses have reported an overall incidence of HFSR across all grades ranging from 30% to 40%, with grade 2 or higher lesions occurring in 8% to 9% of cases. This underscores the need for thorough monitoring and appropriate management of this common side effect in patients undergoing sorafenib treatment.
Gastrointestinal disturbances are commonly observed during sorafenib treatment, manifesting as diarrhea, vomiting, nausea, and loss of appetite. Diarrhea is the most frequently reported symptom among these. Typically, these gastrointestinal side effects are mild, classified as grade 1 or 2; however, more severe effects, classified as higher grades, can disrupt a patient's daily activities and require proper management. Medications such as loperamide can be employed to manage the symptoms. Dose adjustments are generally unnecessary unless severe grade 3 or 4 side effects occur.
Renal toxicity, characterized by proteinuria and acute kidney injury, is a known dose-limiting side effect of sorafenib treatment, although it occurs infrequently. The inhibition of the VEGF pathway and subsequent damage to glomerular capillary endothelial cells are believed to play a role in these complications. Consequently, it is essential to monitor and manage blood pressure during sorafenib treatment to prevent renal complications.
Common side effects typically emerge within the first 4–6 months of treatment and generally resolve after 5–6 months. Effective management during this period is essential, as these effects can significantly affect daily life. Similar side effects have been noted with lenvatinib, another multikinase inhibitor similar to sorafenib. However, the incidence of serious adverse reactions was significantly higher in the lenvatinib group, at 43%, compared to 30% in the sorafenib group [47,48].
Immune checkpoint molecules are pivotal in regulating anti-cancer T-cell responses and are expressed on T-cells, antigen-presenting cells such as dendritic cells and macrophages, and tumor cells. These molecules are essential for naturally suppressing T-cell activity and maintaining self-tolerance. Major inhibitory receptors of immune checkpoints include PD-1, PD-L1, CTLA-4, LAG-3, and TIM-3, while co-stimulatory proteins such as CD25, GITR, and OX40 promote T-cell expansion. Consequently, ICIs that target PD-1, PD-L1, CTLA-4, TIM-3, and LAG-3 have shown significant safety and efficacy in treating HCC. Additionally, immunotherapeutic drugs are not metabolized in the liver, which may lead to predictable pharmacokinetic profiles in patients with cirrhosis [49]. In the treatment of advanced HCC, ICIs have improved survival rates compared to sorafenib, but they are also associated with a spectrum of adverse effects that necessitate careful monitoring. Although the precise mechanisms are not fully understood, these effects are often related to the depletion and exhaustion of regulatory T cells, which play a vital role in maintaining tolerance induced by ICI therapy, especially through CTLA-4 blockade. The depletion of these cells can result in decreased anti-inflammatory cytokines, increased proliferation of CD8+ T cells, and early B-cell alterations, potentially leading to immune-related adverse events. These side effects differ from those associated with traditional chemotherapy, as they can be more unpredictable in their onset and may persist longer. This article will discuss the specific side effects associated with key ICIs, including nivolumab, ipilimumab, atezolizumab, bevacizumab, tremelimumab, and durvalumab, in sequence [50,51].
A recombinant human IgG4 monoclonal antibody, administered intravenously, functions as a PD-1 inhibitor. It binds to the PD-1 receptors on the surface of T cells, thereby restoring their ability to combat cancer cells.
In a phase 3 multinational randomized controlled trial (CheckMate 459), a comparison between nivolumab and sorafenib revealed an overall incidence of adverse events at 70%, with 22% of patients experiencing grade 3 or higher adverse events. The most common side effects were fatigue (15%), pruritus (13%), rash (11%), AST elevation (11%), diarrhea (8%), decreased appetite (6%), nausea (5%), weight loss (1%), and hypertension (1%). Severe adverse events (grade 3 or higher) included AST elevation (6%), diarrhea (1%), and palmar-plantar erythrodysesthesia. Grade 3 or higher adverse events occurred more frequently in the sorafenib group (47% vs. 18%), although mild side effects were similarly distributed between the two groups (48% vs. 44%).
Ipilimumab, a CTLA-4 inhibitor, can be used in combination with nivolumab for treatment. The most commonly reported side effects are pruritus (45%), rash (29%), diarrhea (24%), hypothyroidism (20%), fatigue (18%), adrenal insufficiency (14%), and decreased appetite (12%). Additionally, there was a rare instance of a treatment-related death caused by grade 5 pneumonia.
Among patients treated with atezolizumab/bevacizumab, 98% experienced side effects. Of these, 63% reported grade 3 to 4 side effects, and 7% experienced grade 5 side effects. The most common side effects were hypertension (29.8%), fatigue (20.4%), proteinuria (20.1%), hepatitis (AST elevation, 19.5%), pruritus (19.5%), diarrhea (18.8%), decreased appetite (17.6%), rash (12.5%), and nausea (12.2%). Severe side effects included upper gastrointestinal bleeding and increased risks of cardiotoxicity, thromboembolic stroke, and gastrointestinal perforation associated with bevacizumab.
A meta-analysis was conducted to assess the risk of bleeding in HCC patients undergoing treatment with atezolizumab/bevacizumab. This analysis, which included 28 studies, indicated an overall bleeding incidence of 8.42% (95% CI, 5.72%–11.54%), with grade 5 bleeding occurring in 2.06% of cases (95% CI, 0.56–4.22). Gastrointestinal bleeding, particularly variceal bleeding, was identified as the most common bleeding site, with an incidence of 5.48% (95% CI, 3.98%–7.17%). The incidence of bleeding was found to be 2.11 times higher (95% CI, 1.21–3.66) when compared to treatment with TKIs. Additionally, high body mass index and high albumin-bilirubin grade were significant predictors of bleeding complications [52].
In the HIMALAYA clinical study, 75.8% of the patients experienced side effects, with 25.8% encountering grade 3 to 4 adverse reactions. The most common side effects were diarrhea (26.5%), pruritus (22.9%), rash (22.4%), loss of appetite (17%), fatigue (17%), fever (12.9%), nausea (12.1%), elevated AST (12.4%), and hypothyroidism (10.3%).
There have been reports on the relationship between immune-related adverse events and prognosis. Patients who experienced these immune-related side effects showed an improvement in PFS, although there was no significant difference in overall survival. A meta-analysis revealed that patients who developed skin, gastrointestinal, or endocrine-related side effects following nivolumab treatment exhibited a positive correlation with favorable outcomes. The objective response rate after treatment with nivolumab/ipilimumab was positively correlated with the occurrence of skin or gastrointestinal events, but not with other side effects. However, in cases involving anti-PD-(L)-1 monotherapy or combination therapy, patients who experienced grade 2 or higher treatment-related adverse effects demonstrated improved overall survival (HR, 0.55; 95% CI, 0.34–0.88).
In 2023, a meta-analysis was conducted to compare the side effects of sorafenib and first-line immunotherapy in treating HCC. The analysis revealed that patients with unresectable HCC who were treated with ICIs exhibited a higher incidence of all-grade pruritus. Conversely, those receiving sorafenib faced increased risks of diarrhea and HFSR. There were no significant differences observed in the rates of fatigue, elevated aspartate transaminase levels, rash, hypertension, or decreased appetite [53].
Conclusion
A new paradigm in HCC treatment is emerging, with a particular emphasis on combination therapies such as atezolizumab with bevacizumab, durvalumab with tremelimumab, and various traditional kinase inhibitors. These therapeutic strategies are designed to target the complex biological pathways of liver cancer, aiming to inhibit tumor growth and metastasis, and thereby improve survival rates in cases of advanced liver cancer. Clinicians must be well-versed in the latest treatments for advanced HCC and understand the specific indications for each to recommend the most appropriate therapy for individual patients. Additionally, since ICIs have been associated with previously unobserved side effects, careful attention and monitoring are required.

