Introduction
Scabies is a highly contagious skin disease marked by severe itching, resulting from the infestation of mites within the skin. As of 2017, over 200 million people globally were affected, with a notably high prevalence in tropical and low-income areas [1]. In Korea, scabies was once prevalent, representing about 10% of outpatient visits in some general hospitals until the early 1980s. However, the incidence declined to less than 1% by the 1990s, likely due to strengthened public health measures. Recent years have seen a resurgence of scabies, which is thought to be linked to the increase in long-term care facilities associated with an aging population. In 2010, data from the Health Insurance Review and Assessment Service reported over 50,000 cases, but this number decreased to around 30,000 cases by 2021. Despite this decline, there remains a risk of scabies re-emerging.
To reduce scabies outbreaks, especially in communal living settings like nursing homes, it is crucial to implement preventive measures, ensure early detection, and adopt effective management practices. These steps help mitigate risk factors and curb the spread of the disease. Several countries, including the U.S. and various European nations, have established guidelines for the diagnosis, treatment, and prevention of scabies [2–4]. In Korea, the Korea Disease Control and Prevention Agency (KDCA) has been issuing guidance on scabies prevention and management since 2018 [5,6]. Nevertheless, clinical guidelines developed by dermatology experts with experience in treating scabies are vital to broadly support healthcare providers.
In 2023, the Korean Dermatological Association (KDA) prioritized the eradication of scabies, launching clinical services, educational programs in communal facilities, and public awareness campaigns. To bolster these initiatives, the authors formed a committee within the KDA tasked with developing standardized clinical guidelines for managing scabies. We believe this guideline will serve as a valuable resource for everyone engaged in the treatment and prevention of scabies.
Ethics statement
As this was a literature review study, it did not require approval from an institutional review board. Informed consent was obtained from the patients depicted in the figures for the use of their photographs.
Definition, transmission, and epidemiology
-
- Scabies is a skin infection caused by the scabies mite. It is transmitted primarily through direct skin contact or sexual contact with an infected individual. Transmission can also occur, though less commonly, through indirect contact with contaminated objects.
-
- The number of scabies cases in Korea has steadily declined, from approximately 50,000 in the early 2010s to around 30,000 in 2021. While scabies is more prevalent among older adults and its incidence increases with an aging population, about 17% of cases also occur in individuals under the age of 20.
-
- Factors contributing to the ongoing occurrence of scabies cases include the expansion of elderly care facilities, increasing drug resistance, diagnostic difficulties due to atypical clinical presentations in clean environments or cases of scabies incognito, and a rise in travel from regions where the disease is endemic.
Scabies is a skin infection caused by the mite Sarcoptes scabiei, which belongs to the phylum Arthropoda, subclass Arachnida, order Astigmata, and family Sarcoptidae [7]. This mite parasitizes more than 40 animal species, burrowing into the skin and causing infections in both humans and animals. There are different varieties of scabies mites that infect humans and other animals. In Korea, three types have been identified: S. scabiei var. hominis (human scabies mite), S. scabiei var. canis (dog scabies mite), and S. scabiei var. suis (pig scabies mite). Of these, only S. scabiei var. hominis is transmitted between humans and causes clinical disease, and is commonly referred to as the "scabies mite" in medical practice. Mature female mites measure 0.30–0.45 mm in length and 0.25–0.35 mm in width, while mature male mites are approximately half that size [5]. They are oval and grayish-white, with brown legs and a gnathosoma (mouth parts) that is distinct from the idiosoma (body), which is short, blunt, and round with eight legs. These mites lack eyes and respiratory organs, and feature a distinctive long bristle on the third pair of legs.
The lifecycle of S. scabiei comprises four stages: egg, larva, nymph, and adult [5]. After mating on the skin surface, female mites burrow 1–2 mm into the stratum corneum. Here, they lay an average of 35–50 eggs throughout their 4–6-week lifespan. In contrast, male mites typically die within two days of mating. The eggs hatch into larvae within 4–5 days, develop into nymphs, and reach adulthood within 10–14 days. Scabies mites move at a rate of approximately 2.5 cm per minute on the skin and can survive for 24–36 hours, and up to one week, off the host. They are most active at temperatures above 20°C.
Scabies primarily spreads through direct skin-to-skin or sexual contact with an infected person. It can also spread less commonly through indirect contact with contaminated items such as fabrics, doorknobs, bedding, or furniture. Individuals infected with scabies can transmit the disease during the asymptomatic incubation period and remain contagious until the mites and eggs are eliminated through treatment. Crusted scabies, which is highly contagious, often occurs in communal settings such as nursing homes, long-term care facilities, prisons, and daycare centers. High-risk groups for scabies infection include household members and sexual partners of individuals with scabies. Crusted scabies is more likely to affect those who are immunocompromised, older adults, individuals with physical or mental disabilities, and those in poor conditions or with severe underlying diseases [6].
In Korea, the prevalence of scabies among outpatients at eight general hospitals across six regions, including Seoul, was approximately 2% in the 1960s, rising to 3%–7% in the 1970s, and reaching 10% in the early 1980s. However, by the 1990s, it had declined to below 1% [8]. Data from the Korean Health Insurance Review and Assessment Service show that the number of scabies cases in Korea decreased from 51,331 in 2010 to 29,693 in 2021 (Fig. 1) [9]. During the same period, the age-standardized incidence rate per 100,000 people fell from 97.6 to 43.4. Factors contributing to this decline include improved personal hygiene, heightened awareness of scabies in institutional settings such as nursing facilities, and the social isolation measures and movement restrictions associated with the COVID-19 pandemic.
Scabies was most prevalent among individuals in their 40s, accounting for 16% of cases in 2010–2011. However, from 2012 to 2020, the highest incidence shifted to those in their 50s, with a steady increase in this age group. The higher incidence among older adults can be attributed to factors such as population aging, an increase in long-term care facilities, and relative poverty among older adults [9]. In contrast, among individuals under 20, the rate of scabies declined from 33.5% of all cases in 2010 to 16.9% in 2021. The rise in scabies cases among older adults suggests that infections in younger individuals are likely due to secondary transmission from caregivers, healthcare workers, or family members.
The persistent incidence of scabies can be attributed to several factors, including the expansion of elderly care facilities, increasing drug resistance, challenges in diagnosing atypical or cryptic scabies in clean environments, and increased travel to endemic regions [5,10]. Additionally, the rising trend of group infections in facilities, driven by an aging population and prolonged stays, has emerged as a significant social concern.
Clinical manifestations
-
- The clinical presentation of scabies varies depending on the patient's age, health status, mite load, and route of transmission.
-
- Characteristic skin symptoms of the condition include intense itching that worsens at night, along with the presence of burrows and red bumps in typical areas. These areas often include the interdigital web spaces of the fingers, the inner wrists, and around the navel.
-
- In older adults, immunocompromised persons, or infants, scabies may manifest differently, exhibiting less itching and atypical skin features. These can include involvement of the scalp, face, palms, and soles, as well as hyperkeratotic scaling or nodules.
The clinical manifestations of scabies vary based on factors such as the patient's age, health status, underlying diseases, the number of mites present, the route of transmission, and the time elapsed before diagnosis. While nocturnal itching and the appearance of burrows are common symptoms, infants and older patients with underlying conditions may present with unique and varied symptoms. Many patients experience severe itching that disrupts sleep and diminishes their quality of life [11]. Additionally, they may develop skin complications such as bacterial infections, eczema, or bullous pemphigoid [12]. In rare cases, systemic complications such as glomerulonephritis, vasculitis, lymphadenopathy, and sepsis may arise [13]. Scabies is typically categorized into three types based on its clinical features: classic, crusted, and nodular (Table 1).
The primary symptom of classic scabies is intense itching that worsens at night. An analysis of Korean scabies patients revealed that 76.4% experienced nocturnal itching, 23.6% reported severe itching, and 13.8% had sleep disturbances due to the intense itching. This itching is caused by the direct activity of the mites as well as an immunological reaction, primarily Type IV delayed hypersensitivity, to their digestive secretions, excretions, and eggs [14,15]. The worsening of symptoms at night is linked to increased mite activity, elevated secretion levels, and heightened nerve sensitivity, which occurs due to a decrease in sympathetic nervous activity [14].
The incubation period before the onset of itching typically ranges from 4 to 6 weeks. However, it can be shorter (i.e., less than 4 weeks) when a large number of scabies mites are present [16]. In cases of reinfection, symptoms can manifest within 1 to 3 days [17]. Itching may continue for several weeks even after the mites have been eradicated. The severity of the itching often correlates with the number of scabies lesions.
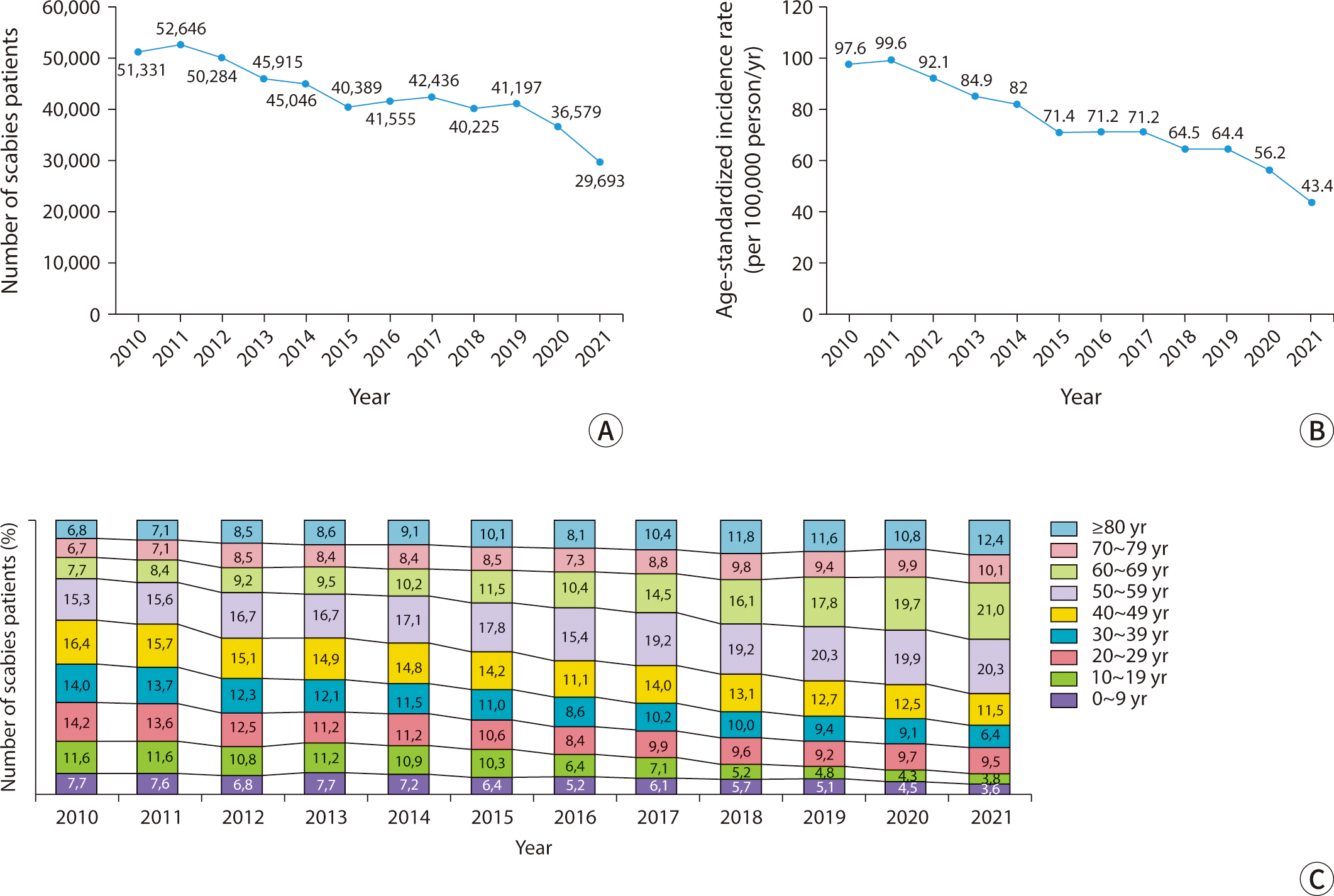
The burrows created by female mites as they tunnel through the stratum corneum are distinctive dermatological manifestations. These burrows typically measure 2–10 mm in length and appear as grayish-white or light brown linear tracks, either thread-like or wavy. Upon close examination, one might notice fine scales on their surface, accompanied by slightly darker or raised edges and ends (Fig. 2A, B) [3]. Commonly, these burrows are located between the interdigital web spaces of the fingers, on the inner wrists, around the navel, buttocks, male genitalia, female breasts, and armpits [2,4]. This distribution pattern indicates the mites' preference for warmer, thinner, and less hairy skin [18]. In approximately 80% of patients, 1 to 4 burrows are found within the same area of skin. Small, erythematous macules and papules, typically 2–3 mm in size and often accompanied by severe itching, are common skin lesions resulting from hypersensitivity to mites (Fig. 2C, D). These lesions are usually observed around the lower abdomen, inner thighs, axillae, and inner arms, although they do not always correspond with the distribution of the burrows [2,17]. In most healthy adults, the scalp, face, palms, and soles are typically unaffected. However, in infants and older adults, one may observe burrows, papules, vesicles, and pustules on the palms and soles [19]. In cases of prolonged infestation, repeated scratching and infection can result in atypical presentations, including excoriations, oozing, and crusted lesions (Fig. 2E, F).
Previously known as Norwegian scabies, crusted scabies primarily affects immunocompromised individuals due to physical or mental disabilities, chronic systemic illnesses, or prolonged steroid use [20]. However, approximately 40% of cases do not have identifiable risk factors [21]. This form of scabies is highly contagious because of the large number of mites present, yet patients typically experience minimal or no itching due to a diminished cellular immune response. It is characterized by extensive, thick hyperkeratotic scales and fissures, which resemble psoriasis or chronic eczema (Fig. 2G). Skin lesions commonly appear on the palms, soles, head, neck, buttocks, elbows, and knees, particularly in areas subjected to friction. Burrows may also be present in non-hyperkeratotic areas. In some instances, the infection can extend to the nails, causing nail deformities, or progress to erythroderma [22–24]. Secondary bacterial infections and lymphadenopathy are common, and patients who are immunocompromised are at an increased risk of developing sepsis [25].
Nodular scabies is a variant of classic scabies, affecting approximately 10%–30% of all cases. It is characterized by intensely itchy, red-brown papules and nodules ranging from 5–20 mm in size. These lesions are typically located on the male genitalia, buttocks, groin, and axillae (Fig. 2H). In infants, nodules can also be found on the trunk, limbs, or even across the entire body. Early in the disease, burrows may occasionally be visible on the surface of these nodules. Even after the mites have been eradicated, the nodules often persist for some time, with 80% resolving within three months, though some may last up to a year [26].
Uncommon variants of scabies include canine scabies and scabies incognito. Canine scabies is caused by S. scabiei var. canis and is transmitted from dogs or other animals. Due to host specificity, it typically resolves spontaneously in humans within a few days and does not spread between humans [27,28]. Scabies incognito occurs when the prolonged use of steroids or other treatments suppresses the immune and inflammatory responses, leading to atypical clinical features [29,30]. Instead of the usual symptoms of itching or burrows, scabies incognito presents with widespread eczema-like lesions, which can result in a delayed diagnosis and the potential for ongoing transmission to others.
Diagnosis
-
- Scabies is diagnosed clinically through a patient's history of contact with others who have scabies and the presence of characteristic skin symptoms. Confirmation of the diagnosis is achieved by identifying mites using microscopy or dermoscopy.
-
- A clinical diagnosis of scabies is possible when burrows are visible or when there is a history of contact with a scabies patient, accompanied by nodules in the genital area or characteristic small papules in typical locations.
-
- Microscopic examination of skin samples provides high specificity. In contrast, dermoscopy is a quick and convenient method that achieves accuracy comparable to microscopy when conducted by experienced clinicians. This technique is particularly advantageous for older patients or infants who may have difficulty cooperating during testing.
The diagnosis of scabies primarily relies on a clinical evaluation, which includes assessing contact history and identifying characteristic skin lesions. Confirmation is achieved through microscopy, dermoscopy, or high-magnification imaging techniques that detect mites [2–4,31]. In many medical institutions in Korea, there is limited access to these mite detection tests; thus, treatment for scabies often begins based solely on the clinical diagnosis [32]. According to a Korean multicenter study, approximately half of the scabies cases were diagnosed clinically without confirmatory testing. While a patient's history and typical skin findings are invaluable for raising suspicion and aiding in the diagnosis when confirmatory tests are unavailable, they cannot fully replace diagnostic tests. Special consideration should also be given to immunocompromised patients, infants, and older adults, who may not exhibit typical clinical symptoms.
Recently, a consensus was reached by 34 global scabies experts on standardized diagnostic criteria for scabies [2,33]. These criteria categorize the diagnosis into three levels based on diagnostic certainty: suspected, clinical, and confirmed. This stratification provides a valuable tool for diagnosing classic scabies in various clinical settings [34]. The sensitivity and specificity of these standardized criteria range from 69% to 83% and 70% to 96%, respectively [33,34]. However, these criteria may be less appropriate for atypical cases, such as crusted scabies. A simplified version of the criteria, presented in Table 2, is also in use, although its accuracy still requires further validation.
Contact history is a crucial factor in diagnosing scabies, as it is observed in most cases. When a patient presents with pruritic skin lesions and has a history of contact with someone diagnosed with scabies, scabies should be suspected. This contact history can include direct interactions with the infected individual, such as those involving cohabitants, family members, sexual partners, roommates, healthcare providers, or caregivers. Additionally, individuals who have had close skin contact or direct exposure to contaminated clothing or bedding are considered to be at high risk (Table 3) [2,5]. Therefore, it is essential to thoroughly investigate contact history, family history, sexual relationships, and any visits or stays in long-term care facilities during the history-taking process. However, not all patients with scabies will have an identifiable contact history, and in some cases, the contact may not yet have been diagnosed with scabies. Furthermore, even with a known contact history, itching and skin lesions may not manifest until the end of the incubation period.
Itching is a common symptom among patients with scabies, though it is nonspecific and alone is not sufficient for diagnosis. This is especially pertinent in older adults, who may experience intense itching as a result of dry skin, medications, or psychological factors. Similarly, immunocompromised individuals or young children might exhibit mild or no itching at all; therefore, the absence of itching should not rule out the possibility of scabies.
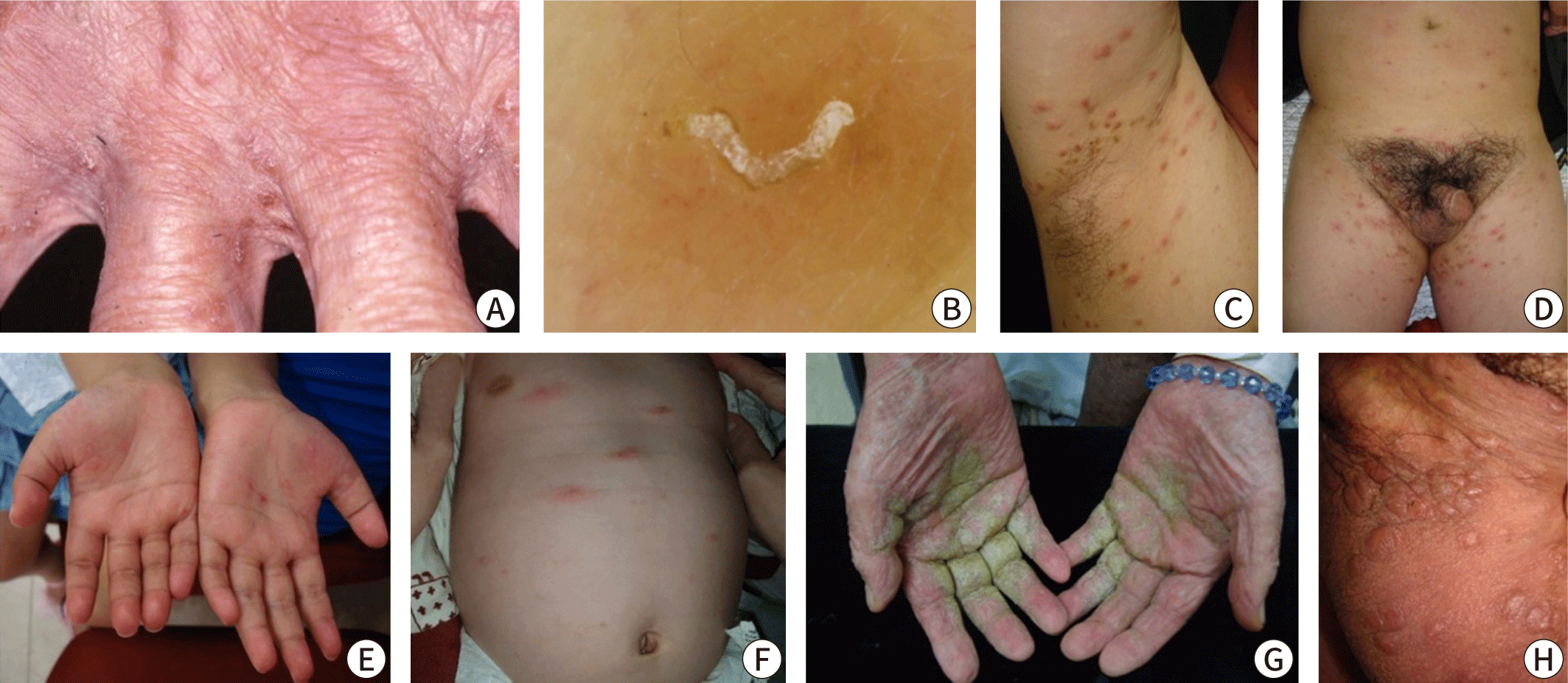
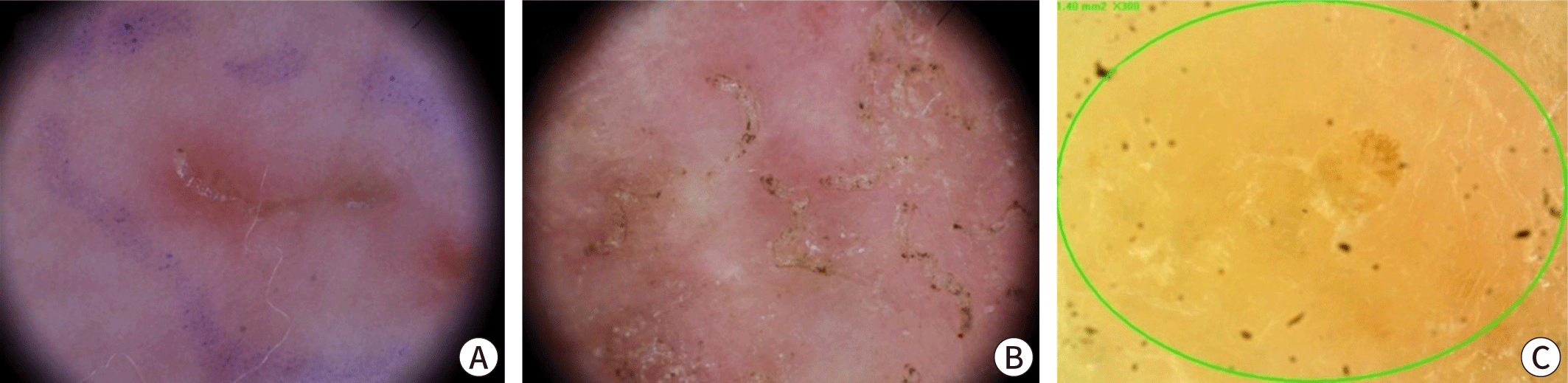
Clinically, scabies is often diagnosed through the observation of burrows during a physical examination or when a patient with a history of contact presents with distinctive skin findings, such as nodular lesions on the male genitalia or pruritic papules in typical locations. Burrows, a hallmark of scabies, are commonly located between the interdigital web spaces of the fingers or on the inner wrists. Although these thread-like lesions might be visible to the naked eye of an experienced physician, early stages of the disease, secondary eczema, or bacterial infections can obscure the burrows. Therefore, it is advisable to thoroughly examine typical sites. In infants, it is also important to check the palms, soles, and scalp. The "burrow ink test" can be helpful in identifying burrows. This test involves rubbing the suspected burrow area with a surgical marker and then removing the excess ink with an alcohol swab (Fig. 3) [35,36]. Erythematous papules with intense itching on the lower abdomen, inner thighs, or nodules on the male genitalia can also support the diagnosis, although these findings are not specific.
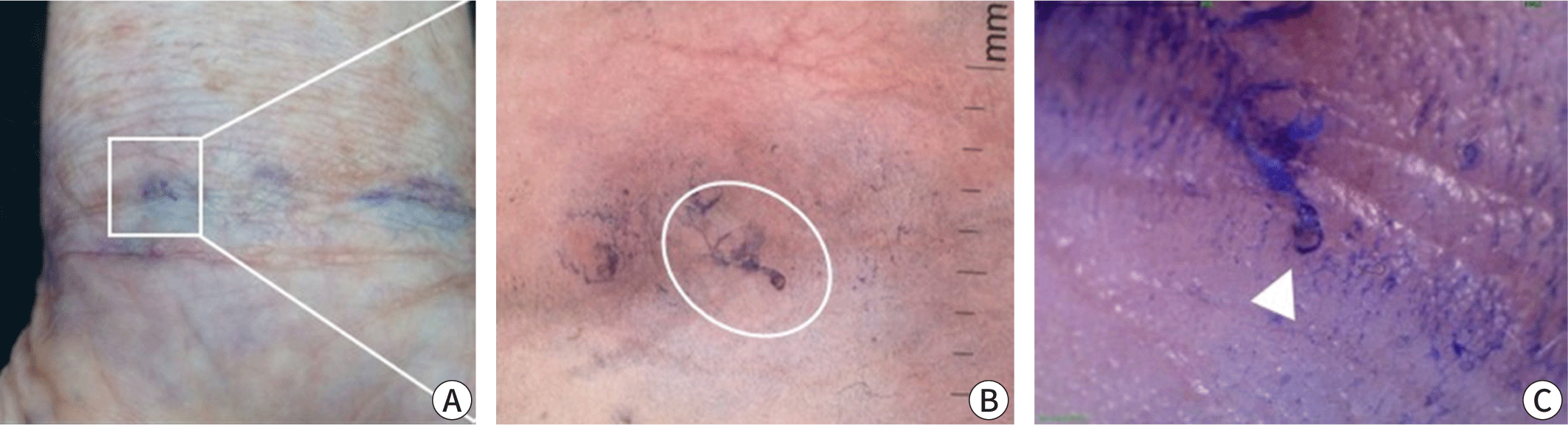
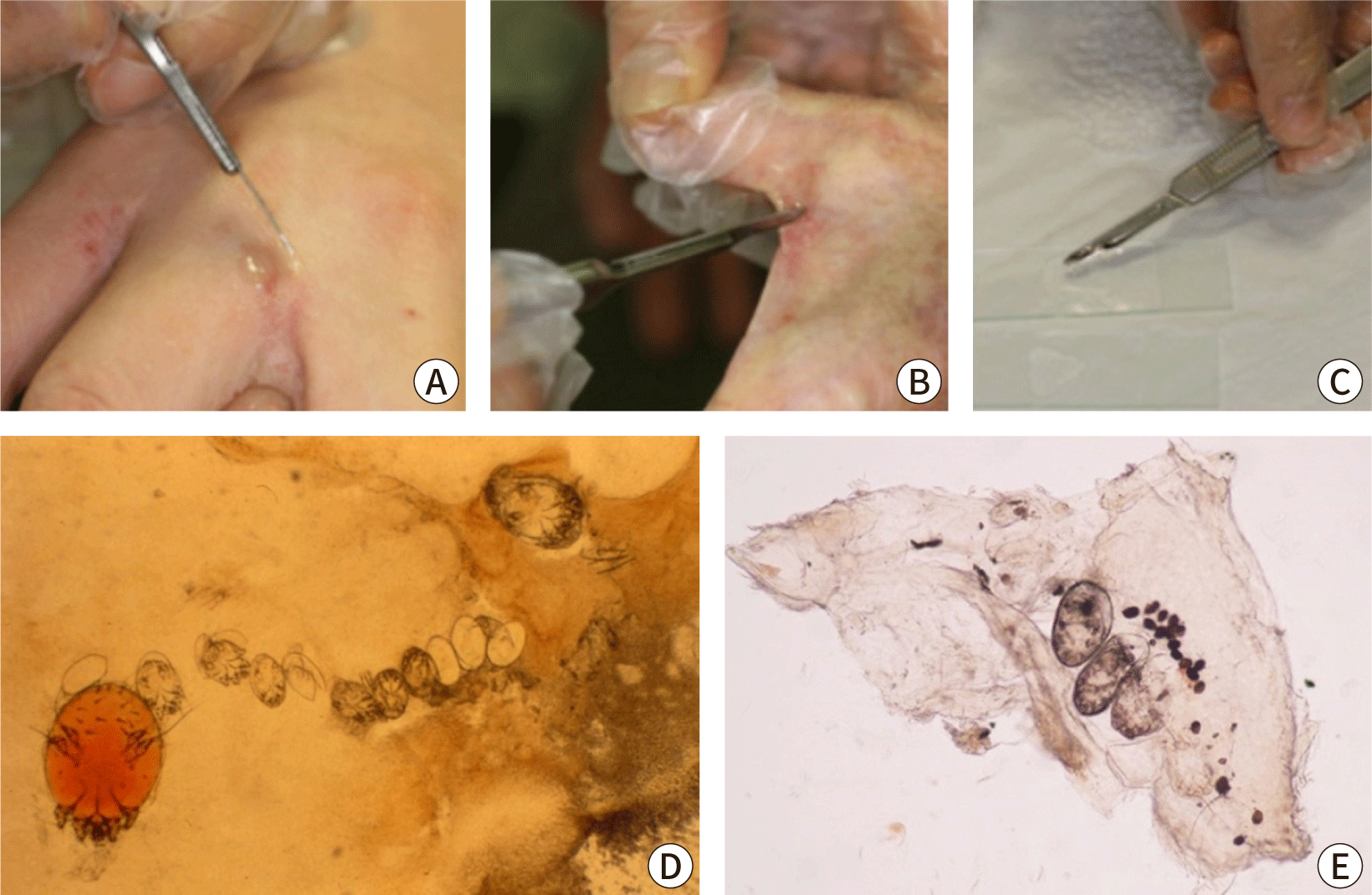
Scabies can be confirmed by visually detecting live mites through a microscopic examination of skin samples, dermoscopy, or other high-magnification imaging methods [2]. For microscopy, skin samples are collected from suspected burrows using a scalpel or adhesive tape, placed on a slide, and observed under a light microscope (Fig. 4). Applying mineral oil to the lesion before sampling helps to capture live mites, eggs, and feces (scybala) [37]. Alternatively, potassium hydroxide can be used to dissolve the keratin or debris in skin samples, which offers a clearer view of mites and eggs but dissolves mite feces. Although microscopy has high specificity, it can be cumbersome and has low sensitivity, with positive detection rates varying from 10% to 70%, depending on the examiner’s skill and sampling site [38]. Sampling from typical burrows without abrasion of eczema on areas such as the finger webs and inner wrists can improve detection rates. A negative result does not exclude scabies if clinical suspicion remains high. In a Korean study, the scabies mite detection rate from a single burrow is around 36%, and only 66.7% of scabies cases have detectable mites [39].
Dermoscopy is becoming increasingly popular as a quick, non-invasive, and convenient diagnostic method. While high magnification (×50 or greater) provides the best accuracy, skilled clinicians are able to detect mites using handheld devices at magnifications of ×10–20 [40]. Typically, dermoscopy reveals a distinctive brown triangular mite head, known as the "delta sign" or "hang glider sign," along with a jet-like burrow filled with bubbles and secretions, which gives a "jet with condensation trail" appearance (Fig. 5) [41,42]. These features are recognized for their high sensitivity and specificity [43–45]. Additional indicators include the wake sign, which shows the trail of mite movement, and the gray-edge line or bluish-white structures that represent mite feces [46]. Dermoscopy is particularly advantageous for patients who might find sample collection distressing, such as older adults, immunocompromised individuals, and infants, as it spares them from unnecessary skin sample collection. However, the detection rate can vary significantly depending on the examiner's expertise and the specific area being observed. Other high-magnification imaging techniques, such as reflectance confocal microscopy and optical coherence tomography, are also capable of identifying mites within the stratum corneum, though these methods are rarely used in clinical practice [47–50].
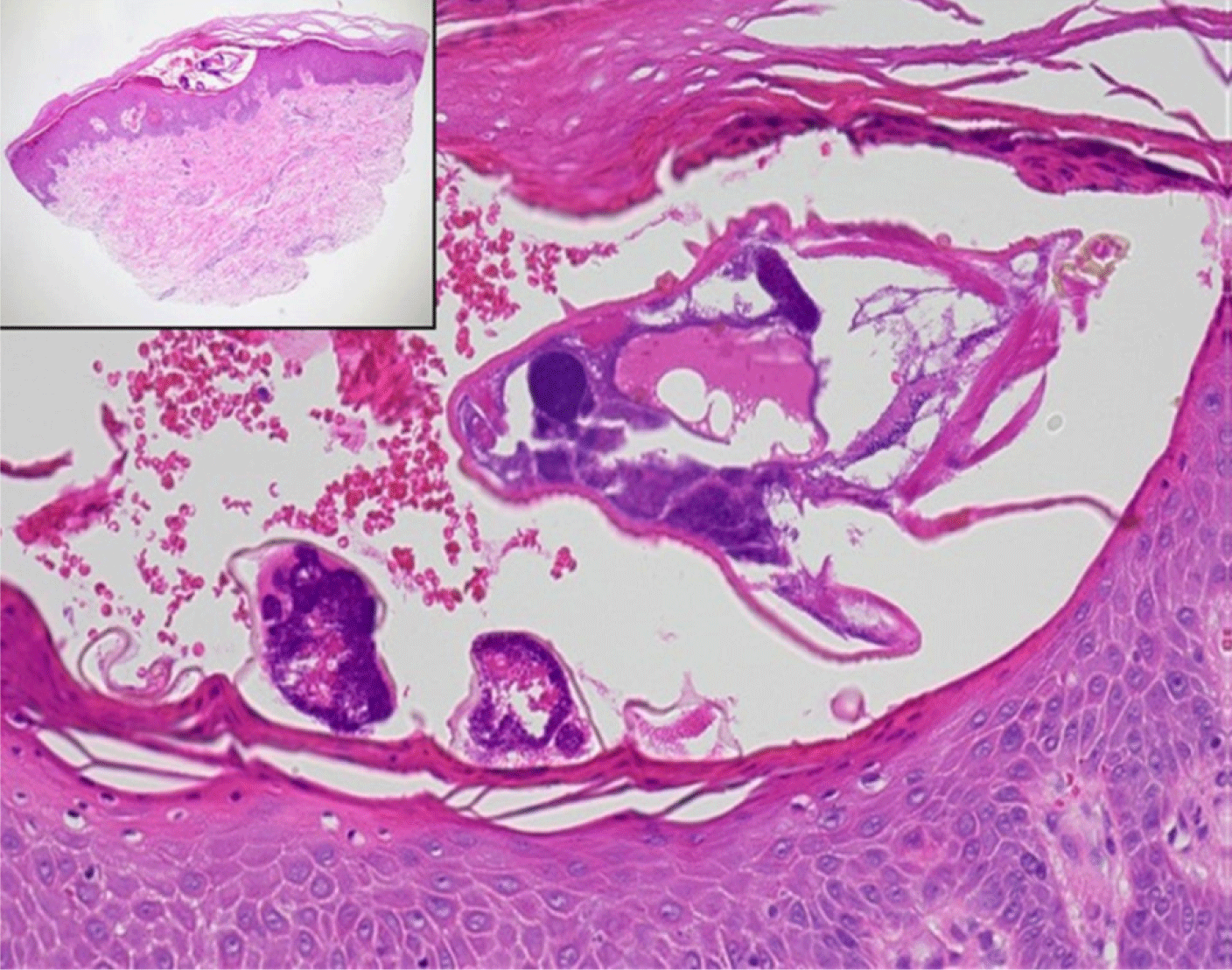
Skin biopsies are generally not performed for diagnosing scabies; however, they can reveal mites, eggs, and feces in the stratum corneum, as well as inflammatory infiltrates, including eosinophils, in the dermis (Fig. 6) [51]. Blood tests do not aid in the diagnosis of scabies. While eosinophils and immunoglobulin E levels may be elevated in patients experiencing itchiness, these elevations are nonspecific. Tests for specific mite antibodies are not commonly used due to their lack of sensitivity and low specificity, which results from cross-reactivity with dust mites [52]. In cases where sexual transmission is suspected or nodules are present in the genital area, testing for sexually transmitted infections, such as syphilis, is advised [53].
Advanced molecular methods are being developed to detect mites. These include nested PCR, reverse transcription-PCR targeting the cox-1 gene, matrix-assisted laser desorption/ionization-time of flight mass spectrometry, and loop-mediated isothermal amplification. However, further research is required to validate these methods for routine clinical use [47,54–56].
Given the nonspecific itching and varied clinical presentations of scabies, it is essential to differentiate it from several other skin conditions (Table 4) [2]. The primary differential diagnoses for classic scabies include insect bites, papular urticaria, atopic dermatitis, contact dermatitis, folliculitis, impetigo, and pityriasis rosea. Delusional parasitosis should be considered in patients who believe in a mite invasion on or inside the skin and repeatedly request diagnostic testing for mite detection without a history of contact. These patients may present objects such as hair, lint, or skin flakes, known as "the matchbox sign," as "proof" of the infestation, despite normal findings on examination. Crusted scabies, characterized by its hyperkeratotic scaling, can closely resemble xerotic eczema, psoriasis, or cutaneous T-cell lymphoma. It may also be mistaken for seborrheic dermatitis when it appears on the head and neck. Nodular scabies may be confused with pseudolymphoma or secondary syphilis, while common lesions on the palms and soles in infants should be differentiated from conditions such as infantile acropustulosis or pompholyx (dyshidrotic eczema).

