| Sang Gyune Kim | 2 Articles |
|
[English]
Citations Citations to this article as recorded by

Special topic: recent management strategies for liver cancer[English]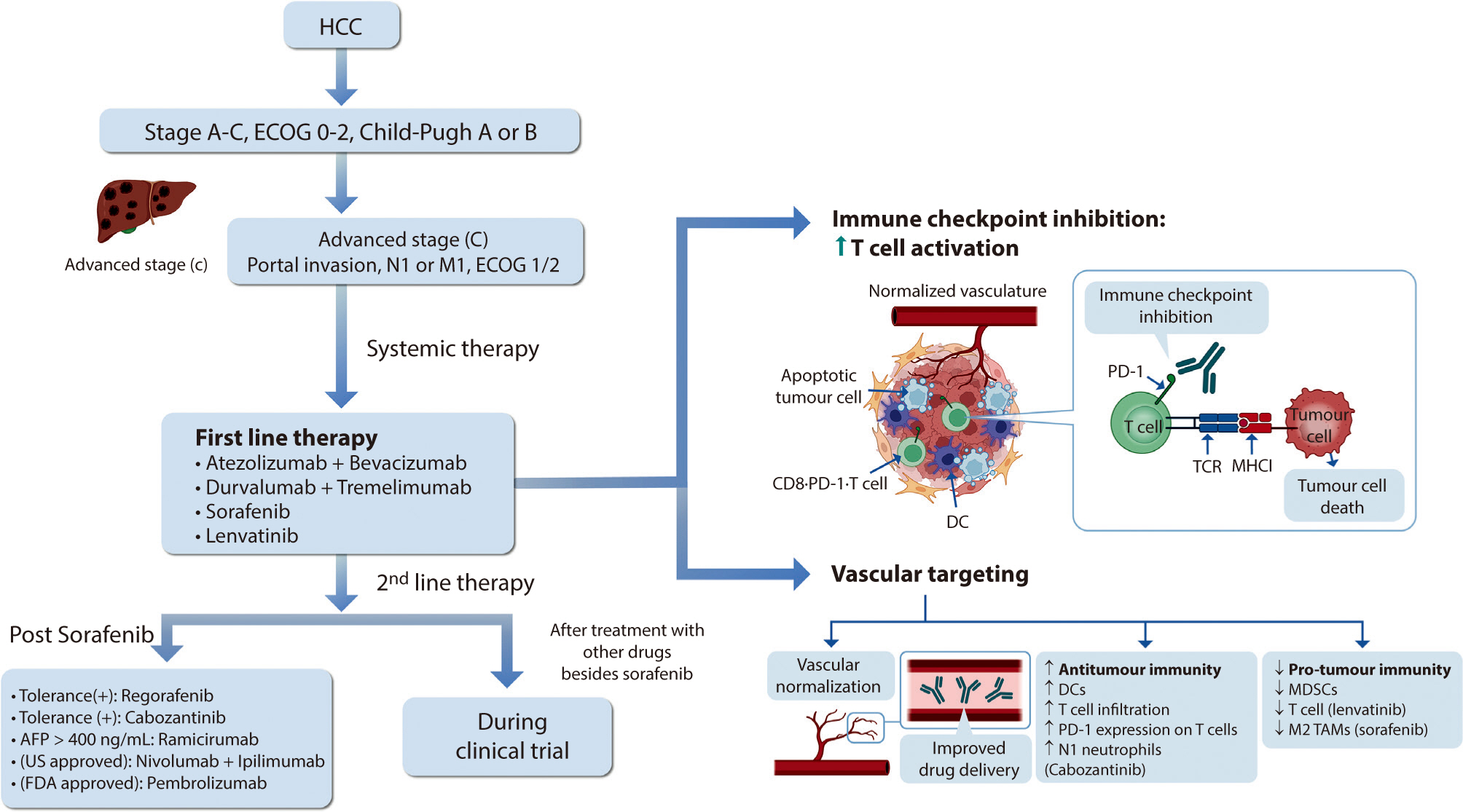
Hepatocellular carcinoma (HCC) remains a critical health concern in Korea, ranking as the second leading cause of cancer mortality and imposing substantial economic burdens, particularly among the working-age population. This review examines recent advancements in treating advanced HCC, referencing the updated 2022 HCC guidelines and the Barcelona Clinical Liver Cancer system. Historically, first-line systemic therapies included sorafenib and lenvatinib, with regorafenib, cabozantinib, or ramucirumab serving as second-line options. Since 2020, immune checkpoint inhibitors have shown superior overall survival than sorafenib, leading to the adoption of combination therapies such as atezolizumab with bevacizumab and durvalumab with tremelimumab as first-line treatments. The IMbrave150 study demonstrated that atezolizumab–bevacizumab significantly extended median overall survival and progression-free survival, with the longest survival reported in any phase 3 trial for advanced HCC. Similarly, the HIMALAYA study indicated that durvalumab combined with tremelimumab significantly improved survival rates. Second-line therapies now include regorafenib, cabozantinib, ramucirumab, nivolumab with ipilimumab, and pembrolizumab, each offering benefits for specific patient populations. Nonetheless, these therapies are associated with side effects that require careful management. Traditional targeted therapies can lead to hypertension, cardiovascular events, and hand-foot skin reactions, whereas immune checkpoint inhibitors may cause immune-related adverse events affecting the skin, gastrointestinal tract, and endocrine system. Clinicians must be well-versed in these treatments and their potential side effects to provide optimal patient care. The emergence of combination therapies targeting complex biological pathways signifies a new paradigm in HCC treatment, emphasizing the importance of continuous education and vigilant monitoring to optimize patient outcomes. Citations Citations to this article as recorded by

|
|

