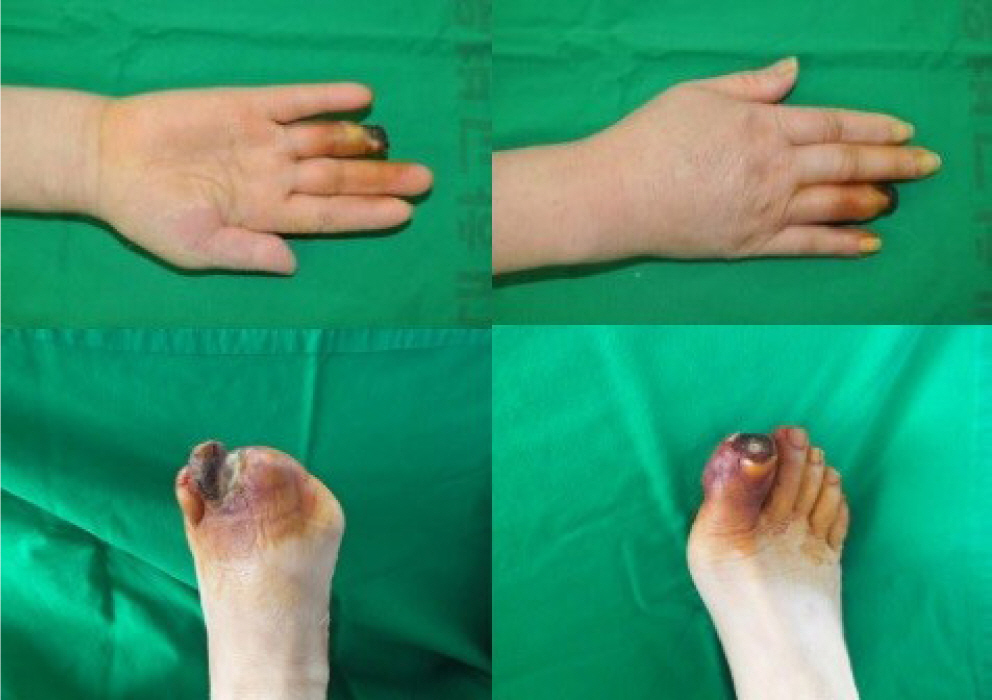
Symmetrical peripheral gangrene is a severe condition marked by symmetric acral necrosis without obstruction of the major blood vessels. This case report examines the critical decisions involved in choosing between early and delayed amputation, as well as determining the extent of the necessary amputation. We present three cases: one involving antiphospholipid syndrome, another with disseminated intravascular coagulation, and a third associated with diabetes mellitus. All three cases ultimately required amputation due to symmetrical peripheral gangrene. In the first two cases, amputation was delayed, which is typically advantageous as it allows for the clear demarcation of necrotic tissue. However, in the third case, where infection was evident, immediate amputation was necessary despite the patient's overall poor health.

Sepsis and its associated complication of disseminated intravascular coagulation (DIC) are considered to be a major cause of morbidity and mortality in the newborn infants. Antithrombin(AT) is a single chain glycoprotein in plasma and belongs to the family of the serpins. It is an important anticoagulant protein acting as a heparin cofactor. However, it's effects and action in preterm infants are not clearly defined. The objective of this study was to determine whether AT III was effective in treatment of DIC in the premature infants.
We studied 52 preterm infants with clinical and laboratory diagnosis of DIC from November 1998 to October 1999. We examined the AT III, platelet, prothrombin time(PT), activated partial thromboplastin time(aPTT), fibrinogen, fibrinogen degradation product(FDP), and D-dimer before and after the treatment with AT III.
1) AT III concentrates were administerd for a mean of 3.6 days. Plasma AT III concetrations were elevated significantly(
2) Hematologic data(PT, aPTT, Fibrinogen, FDP) was significantly improved in definitive DIC group after AT III treatment(
3) The incidence of complications of DIC patients were slightly higher in definitive DIC group than in suspected DIC group, but there was no statistically significant difference. And AT III concentrate were well tolerated in all patients.
This data suggested that AT III therapy was effective to improve the clinical and laboratory findings without significant side effects in the premature infants with DIC.

