
 , Jeong-Ju Yoo
, Jeong-Ju Yoo , Sang Gyune Kim
, Sang Gyune Kim , Young Seok Kim
, Young Seok Kim
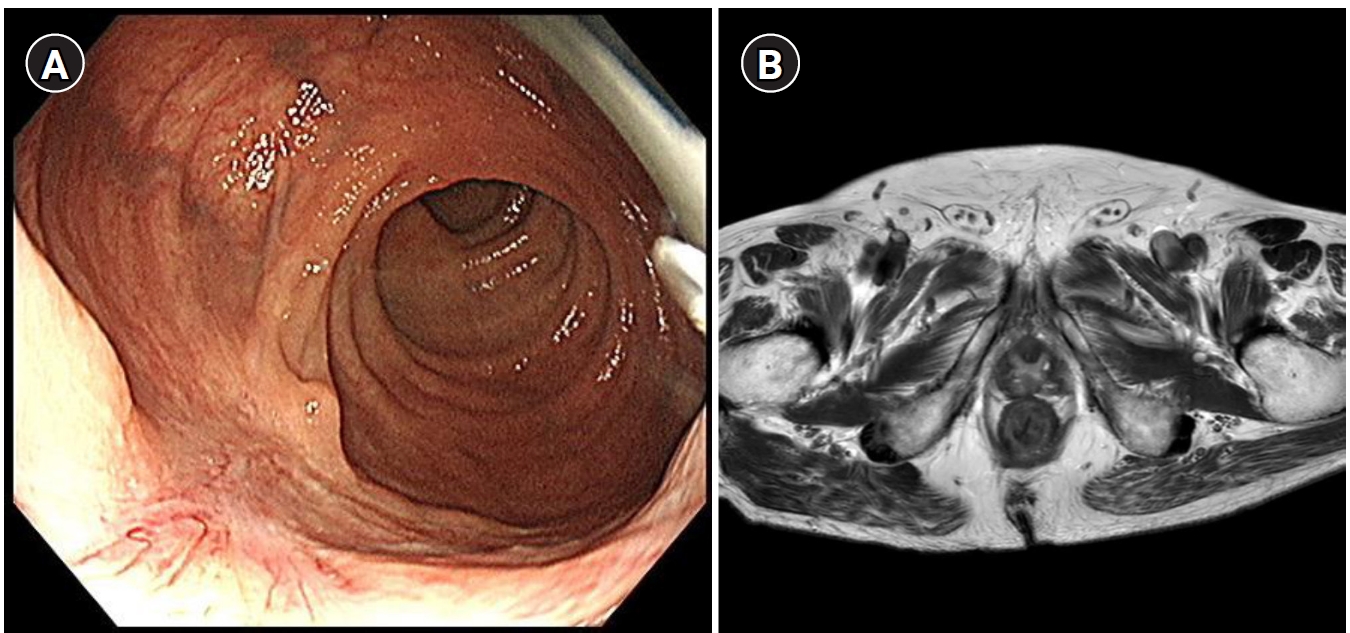
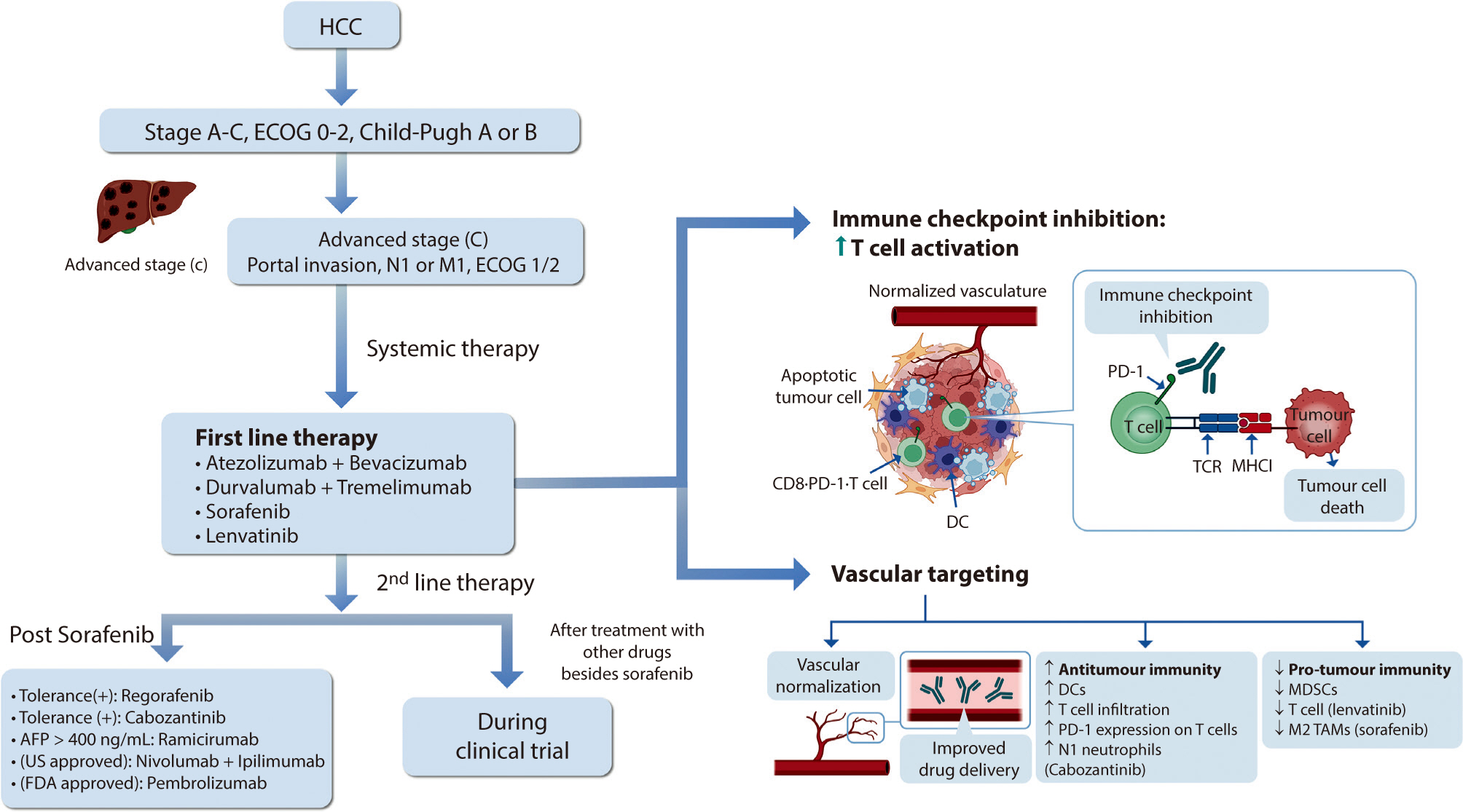
Hepatocellular carcinoma (HCC) remains a critical health concern in Korea, ranking as the second leading cause of cancer mortality and imposing substantial economic burdens, particularly among the working-age population. This review examines recent advancements in treating advanced HCC, referencing the updated 2022 HCC guidelines and the Barcelona Clinical Liver Cancer system. Historically, first-line systemic therapies included sorafenib and lenvatinib, with regorafenib, cabozantinib, or ramucirumab serving as second-line options. Since 2020, immune checkpoint inhibitors have shown superior overall survival than sorafenib, leading to the adoption of combination therapies such as atezolizumab with bevacizumab and durvalumab with tremelimumab as first-line treatments. The IMbrave150 study demonstrated that atezolizumab–bevacizumab significantly extended median overall survival and progression-free survival, with the longest survival reported in any phase 3 trial for advanced HCC. Similarly, the HIMALAYA study indicated that durvalumab combined with tremelimumab significantly improved survival rates. Second-line therapies now include regorafenib, cabozantinib, ramucirumab, nivolumab with ipilimumab, and pembrolizumab, each offering benefits for specific patient populations. Nonetheless, these therapies are associated with side effects that require careful management. Traditional targeted therapies can lead to hypertension, cardiovascular events, and hand-foot skin reactions, whereas immune checkpoint inhibitors may cause immune-related adverse events affecting the skin, gastrointestinal tract, and endocrine system. Clinicians must be well-versed in these treatments and their potential side effects to provide optimal patient care. The emergence of combination therapies targeting complex biological pathways signifies a new paradigm in HCC treatment, emphasizing the importance of continuous education and vigilant monitoring to optimize patient outcomes.
Citations

 , In Sook Kang
, In Sook Kang , Kihwan Kwon
, Kihwan Kwon , Wook Bum Pyun
, Wook Bum Pyun , Gil Ja Shin
, Gil Ja Shin
This study designed to find the differences of left ventricular (LV) geometry in acute myocardial infarction (AMI) between ST elevation myocardial infarction (STEMI) and non ST elevation myocardial infarction (NSTEMI) and the occurrences of adverse outcome according to the LV geometry.
Comprehensive echocardiographic analyses were performed in 256 patients with AMI. The left ventricular mass index (LVMI) and relative wall thickness (RWT) were calculated. LV geometry were classified into 4 groups based on RWT and LVMI: normal geometry (normal LVMI and normal RWT), concentric remodeling (normal LVMI and increased RWT), eccentric hypertrophy (increased LVMI and normal RWT), and concentric hypertrophy (increased LVMI and increased RWT). Cox proportional hazards models were used to evaluate the relationships among LV geometry and clinical outcomes.
Patients with NSTEMI were more likely to have diabetes mellitus, hypertension, heart failure, stroke and previous myocardial infarction. By the geometric type, patients with NSTEMI were more likely to have eccentric hypertrophy (n=51, 34.7% vs. n=24, 22.0%, P=0.028). There was no significantly different adverse outcome between STEMI and NSTEMI patients. Fifteen patients (5.9%, 7 female [46.7%]) died and the median duration of survival was 10 days (range, 1 to 386 days). Concentric hypertrophy carried the greatest risk of all cause mortality (hazard ratios, 5.83; 95% confidence interval, 1.04 to 32.7).
NSTEMI patients had more likely to have eccentric hypertrophy but adverse outcome after AMI was not different between STEMI and NSTEMI patients. Concentric hypertrophy had the greatest risk of short term mortality.
 , Ki Sook Hong
, Ki Sook Hong
Cardiac troponin T(cTnT) levels are elevated in patients with chronic renal fail-ure(CRF) with dialysis which represent myocardial damage. But the cut-off levels were different in laboratories and clinical physicians. We conducted a study to find out the cut-off levels of acute myocardial infarction(AMI), ischemic heart disease(IHD), and cardiovascular disease (CVD) in CRF patients with dialysis and prognostic aspect according to cTnT levels.
Cardiac troponin T(cTnT) of total 98 patients(men 43, women 55, mean age 60.4±13.0 years) was reviewed the diagnosis and progress for 3 years by the medical records. Serum cTnT by Elecsys 2010(Roche diagnostics, Germany), the 4th generation assay was performed.
Mean cTnT level of total 98 patients was 0.26ng/mL and the patients with CVD were 59(60.2%) and their cTnT level was 0.41 ng/mL. The mean levels of cTnT in AMI, IHD, and CVD were 1.10, 0.52, and 0.41 ng/mL, respectively. cTnT, CK, CK-MB, and glucose were increased according to severity of cardiovascular disease. The cut-off levels of cTnT in AMI, IHD, and CVD was 0.10, 0.07 and 0.06 ng/mL. The sensitivity and specificity of AMI, IHD, and CVD in each cut-off level were 88.2/71.6%, 76.2/71.4%, and 81.4/71.8%, respectively. The survival rate above cTnT 0.1 ng/mL during 3 years was significantly decreased(p<0.001) than less than 0.1 ng/mL.
The degree of cTnT elevation in CRF patients with dialysis represents severity of cardiovascular disease and poor survival rate.

