Department of Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
Copyright © 2014. Ewha Womans University School of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
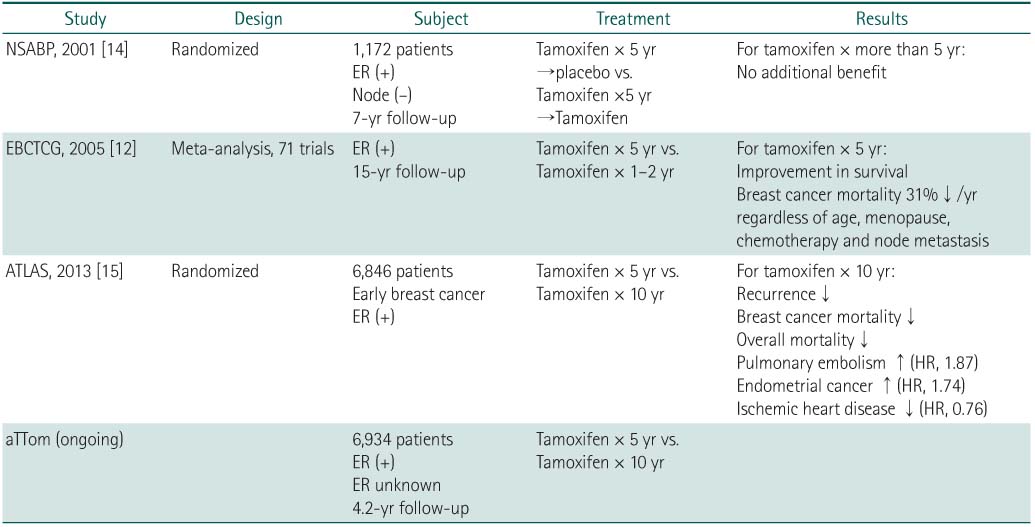
NSABP, National Surgical Adjuvant Breast and Bowel Project; ER, estrogen receptor; EBCTCG, Early Breast Cancer Trialists' Collaborative Group; ATLAS, Adjuvant Tamoxifen Longer Against Shorter; aTTom, adjuvant Tamoxifen Treatment offers more.
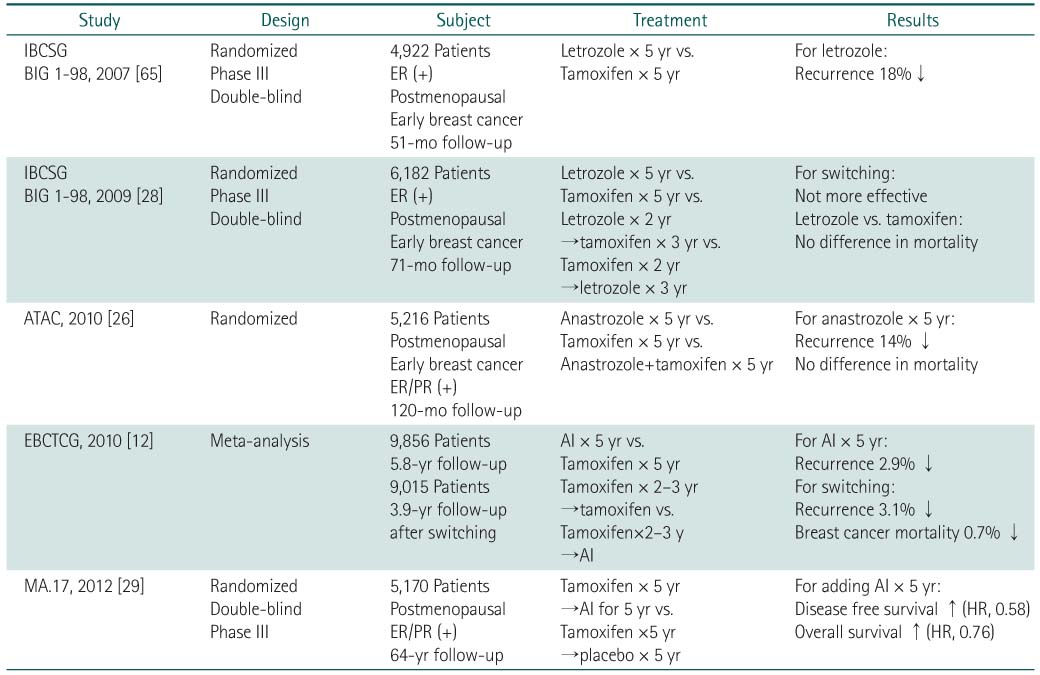
IBCSG, International Breast Cancer Study Group; BIG, Breast International Group; ER, estrogen receptor; ATAC, arimidex, tamoxifen, alone or in combination; PR, progesterone receptor; EBCTCG, Early Breast Cancer Trialists' Collaborative Group; AI, aromatase inhibitor.
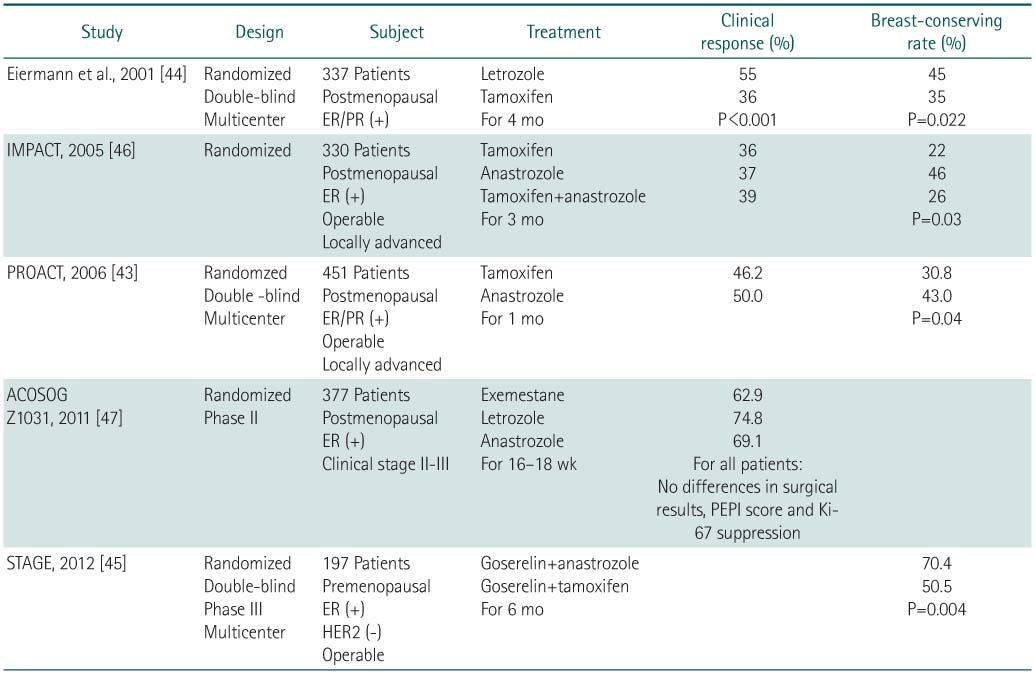
ER, estrogen receptor; PR, progesterone receptor; IMPACT, immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen; PROACT, pre-operative "arimidex" compared to tamoxifen; ACOSOG, American college of surgeons oncology group; PEPI, Preoperative endocrine prognostic index.

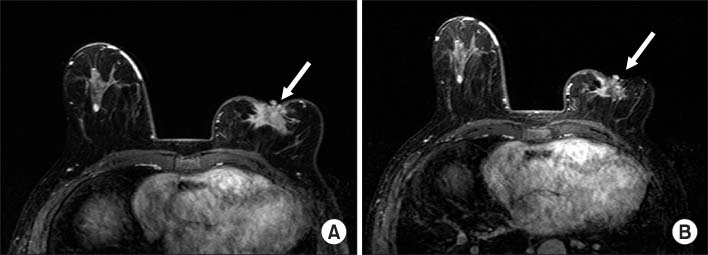
A summary of research studies on adjuvant tamoxifen
NSABP, National Surgical Adjuvant Breast and Bowel Project; ER, estrogen receptor; EBCTCG, Early Breast Cancer Trialists' Collaborative Group; ATLAS, Adjuvant Tamoxifen Longer Against Shorter; aTTom, adjuvant Tamoxifen Treatment offers more.
A summary of research studies on adjuvant aromatase inhibitor
IBCSG, International Breast Cancer Study Group; BIG, Breast International Group; ER, estrogen receptor; ATAC, arimidex, tamoxifen, alone or in combination; PR, progesterone receptor; EBCTCG, Early Breast Cancer Trialists' Collaborative Group; AI, aromatase inhibitor.
A summary of research studies on neoadjuvant endocrine therapy
ER, estrogen receptor; PR, progesterone receptor; IMPACT, immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen; PROACT, pre-operative "arimidex" compared to tamoxifen; ACOSOG, American college of surgeons oncology group; PEPI, Preoperative endocrine prognostic index.
NSABP, National Surgical Adjuvant Breast and Bowel Project; ER, estrogen receptor; EBCTCG, Early Breast Cancer Trialists' Collaborative Group; ATLAS, Adjuvant Tamoxifen Longer Against Shorter; aTTom, adjuvant Tamoxifen Treatment offers more.
IBCSG, International Breast Cancer Study Group; BIG, Breast International Group; ER, estrogen receptor; ATAC, arimidex, tamoxifen, alone or in combination; PR, progesterone receptor; EBCTCG, Early Breast Cancer Trialists' Collaborative Group; AI, aromatase inhibitor.
ER, estrogen receptor; PR, progesterone receptor; IMPACT, immediate preoperative anastrozole, tamoxifen, or combined with tamoxifen; PROACT, pre-operative "arimidex" compared to tamoxifen; ACOSOG, American college of surgeons oncology group; PEPI, Preoperative endocrine prognostic index.