Abstract
In response to the changes in the Coronavirus disease 2019 (COVID-19) epidemic
situation, Ewha Womans University established Ewha Safe Campus (ESC), an
on-campus infection outbreak management system, to allow students and faculty
members to safely resume face-to face classes in 2022. The COVID-19 testing
station, Ewha Safe Station, is the core element of ESC. Symptomatic students and
faculty members perform a combo swab self-PCR test or receive a nasopharyngeal
swab PCR test from experts to prevent the spread of COVID-19 through early
detection and management. ESC is significant in that it detects infection risks
and proactively implements preemptive measures in a university. The COVID-19
health response system model at the university level was applied for the first
time in South Korea, reaching a milestone in the history of university health in
South Korea. In particular, it is highly valuable that the test was free of
charge, as it enabled all of the examinees to have easy access to the test
through joint cooperation with the Seegene Medical Foundation. This is a
successful example of cooperation between schools and private institutions for
public health improvement. In the future, the direct and indirect effects of the
establishment and implementation of ESC need to be evaluated and confirmed, and
areas requiring improvements need to be identified in preparation for another
infectious disease outbreak in the future.
-
Keywords: COVID-19; Ewha Safe Campus; Ewha Safe Station; Nasal swab
Background
On March 11, 2020, the World Health Organization (WHO) declared the Coronavirus
disease 2019 (COVID-19) a global pandemic [
1].
As of January 31, 2022, the global cumulative numbers of confirmed cases and deaths
of COVID-19 reached 376,854,195 and 5,695,057, respectively [
2]. The first case of COVID-19 infection in South Korea was
reported in January 2020 [
3]. By the end of
January 2022, there had been four epidemics in South Korea. As of January 31, 2022,
the cumulative numbers of confirmed cases and deaths in South Korea were 845,610 and
6,755, respectively [
4]. As the pandemic
continued, the virus mutated, which affected South Korea. In the fourth epidemic,
which lasted about six months starting in July 2021 in South Korea, the Delta
variant was the major cause [
3]. In November
2021, the South Korean government implemented Living with COVID, a phased recovery
to “normal daily lives” [
3]. In
the end of January 2022, the Omicron variant, which is considered to have a higher
transmission rate and lower severity rate than previous variants, spread. Living
with COVID was maintained, with high vaccination rates and treatment dissemination
[
3]. In March 2022, the South Korean
government requested the establishment of an autonomous preventative measure system
for universities to mark the beginning of the semester which coincided with the
fifth epidemic caused by the Omicron variant [
5].
Establishment of the Ewha Safe Campus at Ewha Womans University
In response to the changes in the COVID-19 epidemic situation, Ewha Womans University
(EWU) established Ewha Safe Campus (ESC), an on-campus infection outbreak management
system, to allow students and faculty members to safely resume face-to face classes
in 2022. The COVID-19 testing station, Ewha Safe Station (ESS), is the core element
of ESC. Symptomatic students and faculty members perform a combo swab (nasal and
oral) self-PCR test or receive a nasopharyngeal swab (NPS) PCR test from experts to
prevent the spread of COVID-19 through early detection and management.
1. The executive committee for Ewha Safe Campus (ESC)
With the Vice President of the EWU Office of General Administration as the
chairperson, the executive committee for the establishment of ESC is comprised
of the Medical School, Medical Center, Office of Faculty & Academic
Affairs, Office of Student Affairs, Office of General Administration, Office of
Facilities Management, Office of Information and Communications, Office of
University Relations and Development, Office of Communications, and University
Health Service Center. The executive committee divided and coordinated the work
of each participating department and supervised the overall implementation of
ESC.
2. Establishment of a cooperative system with external organizations
The tests were conducted free of charge under cooperation with the Seegene
Medical Foundation, a specialized molecular diagnostics company that provided
the diagnostic kit supply, which is essential for ESC operation. Unlike NPS
specimen, which is a sample collection method for COVID-19 diagnosis that is
considered to be the gold standard, but is uncomfortable and requires collection
by professional, combo swabs have the advantage of minimal pain (minimally
invasive) by self-collecting both nasal swab (scrubbing the inner surface of the
nostril with a cotton swab) and oral swab (scraping the inner surface of the
mouth with a cotton swab).
In addition to NPS, the US Centers for Disease Control and Prevention (CDC)
permits the use of numerous other upper respiratory specimens, such as
oropharyngeal swab, nasal swab, saliva, and nasal wash [
6]. Numerous prior publications indicate that saliva is a
suitable alternative specimen for COVID-19 diagnosis [
6–
9] and that
combination specimens such as oropharyngeal swab and nasal swab have diagnostic
performance comparable to NPS [
7,
10].
Furthermore, a cooperative system was established with Seodaemun Public Health
Center in Seodaemun District Office for immediate reporting of ESS test results.
The immediate reports were then systemized to enable prompt notification, basic
epidemiological investigation, and management of the confirmed cases.
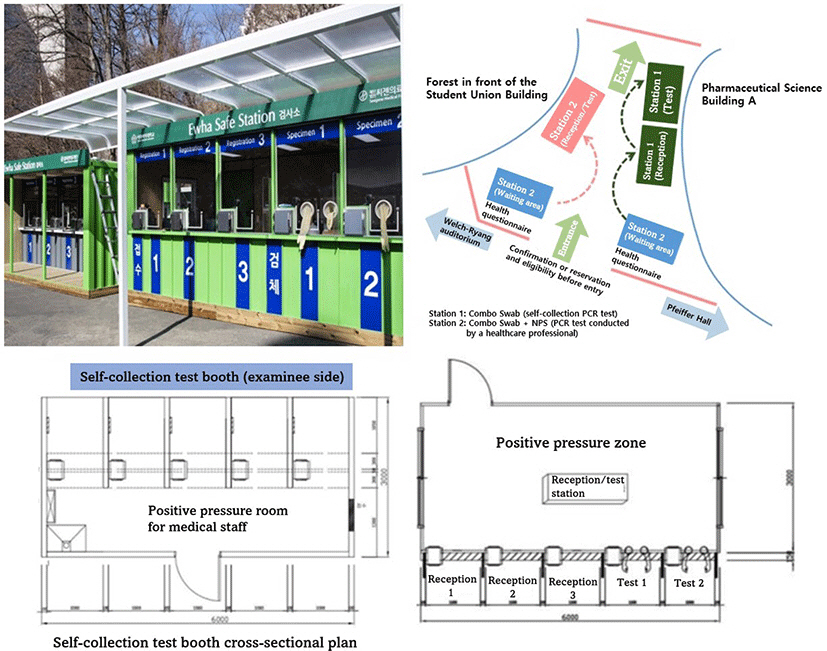
3. Installation and operation of Ewha Safe Station (ESS)
Installed on the EWU campus, the ESS was piloted on February 22, 2022, and the
main test started on March 2nd (
Fig. 1) and
included students and all faculty members, including staff from service
companies (Description of test stations 1 and 2).
Fig. 1.The EWU COVID-19 testing areas (ESSs). The map of Ewha Safe Stations.
The combo swab testing area and symptomatic testing area were separately
operated. COVID-19, Coronavirus disease 2019.
After the executive committee discussion, the subjects were defined as
follows:
Mandatory testing was required for those who were symptomatic, those who
had close contact with confirmed cases, and students in dormitories.
Testing was highly recommended to those who used crowded facilities such
as classes with potential droplet exposure, research facilities, the
library, examination preparation classes, and the gym.
Those who tested negative but showed symptoms such as a fever over
37.5°C were required to get re-tested after three days.
When there was a confirmed case, an epidemiological investigation was
conducted to identify the mobility trends so the EWU Daily Life Recovery
Support Headquarters could inform individual students via text messages.
Testing was mandatory for those who had overlapped mobility trends with
confirmed cases. Furthermore, for the early detection of asymptomatic
patients, weekly tests for vaccinated students and biweekly tests for
unvaccinated students were required.
In the ESC, patients with respiratory symptoms were collected Combo Swabs and NPS
samples simultaneously, whereas subjects without respiratory symptoms were only
collected Combo Swabs. The patient self-collected Combo Swabs under the
observation of the health care experts, while the health care experts collected
NPS. Patients who are symptomatic, have had close interactions or shared social
activities with confirmed patients, and are positive in the Combo Swab test must
take both the Combo Swab and the NPS test at the same time during their ESC
visit. In all other instances, just the Combo Swab test was performed. The
former should take the examination at ESS 2 while the latter should take it at
ESS 1, hence reducing contact between subject groups with a high risk of
confirmation and those with a relatively low risk.
All exams must be scheduled using Eureka's reservation system (Ewha Womans
University Portal). 15-min reservations are restricted to 30 guests. Patients
with abrupt onset of symptoms, however, can undergo an on-site test without
reservation. The ESC was operational on weekdays from 9 a.m. to 4:30 p.m. for a
total of 6 hours and 30 min, omitting one hour for lunch. The test results were
communicated through text message before 7 p.m. on the same day or before 10
a.m. the next day. If the test result is positive, the appropriate public health
facility is contacted and the subjects are instructed to take follow-up
measures, including limits on school and any outside activities. If the
student's enrollment was verified, she may seek a make-up class.
Conclusion and Recommendations
Although the elderly have high ratios of severe COVID-19 and death due to COVID-19,
the young and middle-aged, who are socially active, have relatively high infection
risks [
11]. With active face-to-face contact,
preventative measures need to be proactively prepared in universities. ESC from EWU
is significant in that it detects infection risks and proactively implements
preemptive measures in a university. As one of the factors that contributed to South
Korea’s successful COVID-19 response, early detection testing was applied at
the university level, and the COVID-19 health response system model was applied for
the first time in South Korea, reaching a milestone in the history of university
health in South Korea. In particular, it is highly valuable that the test was free
of charge, as it enabled all of the examinees to have easy access to the test
through joint cooperation with the Seegene Medical Foundation. This is a successful
example of cooperation between schools and private institutions for public health
improvement. In the future, the direct and indirect effects of the establishment and
implementation of ESC need to be evaluated and confirmed, and areas requiring
improvements need to be identified in preparation for another infectious disease
outbreak in the future.
Acknowledgements
This work was supported by the Ewha Womans University Research Grant of 2022 and the
Seegene Medical Foundation.
Conflict of Interest
-
No potential conflict of interest relevant to this article was reported.
Author Contribution
-
Conceptualization: Jung-Choi K, Sung N, Lee SH, Chang M, Choi HJ, Kim CJ, Choi
NK, Kim H, Kim YJ, Lee W, Park H, Ha E
Project Administration: Kim H, Kim YJ
Writing – Original Draft: Jung-Choi K
Writing – Review & Editing: Jung-Choi K, Sung N, Lee SH, Chang M,
Choi HJ, Kim CJ, Choi NK, Kim H, Kim YJ, Lee W, Park H, Ha E
Ethics Approval and Consent to Participate
-
Not applicable.
References
- 1. World Health Organization. Timeline: WHO's COVID-19 response [Internet]. Geneva (CH): World Health Organization; c2022 cited 2022 Sep
23. Available from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline
- 2. World Health Organization. WHO COVID-19 dashboard [Internet]. Geneva (CH): World Health Organization; c2020 cited 2022 Sep
23. Available from https://covid19.who.int/
- 3. Korea Disease Control and Prevention Agency. Public Health Weekly Report: Korea Disease Control and Prevention Agency
[Internet]. Cheongju (KR): Korea Disease Control and Prevention Agency; c2022 cited 2022 Sep
23. Available from https://www.kdca.go.kr/board/board.es?mid=a30501000000&bid=0031&cg_code=C06
- 4. Korea Disease Control and Prevention Agency. COVID-19 [Internet]. Cheongju (KR): Korea Disease Control and Prevention Agency; c2019 cited 2022 Jan
31. Available from https://ncov.kdca.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_id=&gubun=
- 5. Ministry of Education. Ministry of Education [Internet]. Sejong (KR): Ministry of Education;; c2022 cited 2022 Oct
13. Available from https://www.moe.go.kr/boardCnts/viewRenew.do?boardID=294&boardSeq=90598&lev=0&searchType=null&statusYN=W&page=30&s=moe&m=020402&opType=N
- 6. Lee RA, Herigon JC, Benedetti A, Pollock NR, Denkinger CM. Performance of saliva, oropharyngeal swabs, and nasal swabs for
SARS-CoV-2 molecular detection: a systematic review and
meta-analysis. J Clin Microbiol 2021 59(5):e02881-20.
- 7. Nasiri K, Dimitrova A. Comparing saliva and nasopharyngeal swab specimens in the
detection of COVID-19: a systematic review and meta-analysis. J Dent Sci 2021;16(3):799-805.
- 8. Takeuchi Y, Furuchi M, Kamimoto A, Honda K, Matsumura H, Kobayashi R. Saliva-based PCR tests for SARS-CoV-2 detection. J Oral Sci 2020;62(3):350-351.
- 9. Moraleda C, Domínguez-Rodríguez S, Mesa JM, García-Sánchez P, de la Serna M, Alonso-Cadenas JA, et al. Oral saliva swab reverse transcription PCR for Covid-19 in the
paediatric population. Arch Dis Child 2022;1-8.
- 10. Wehrhahn MC, Robson J, Brown S, Bursle E, Byrne S, New D, et al. Self-collection: An appropriate alternative during the SARS-CoV-2
pandemic. J Clin Virol 2020;128:104417
- 11. Salvatore PP, Sula E, Coyle JP, Caruso E, Smith AR, Levine RS, et al. Recent increase in COVID-19 cases reported among adults aged
18–22 years — United States, May 31–September 5,
2020. MMWR Morb Mortal Wkly Rep 2020;69(39):1419-1424.
Figure & Data
Citations
Citations to this article as recorded by

- A Proactive Testing Strategy to COVID-19 for Reopening University
Campus during Omicron Wave in Korea: Ewha Safe Campus (ESC)
Project
Whanhee Lee, Kyunghee Jung-Choi, Hyunjin Park, Seunghee Jun, Nackmoon Sung, Sun-Hwa Lee, Misun Chang, Hee Jung Choi, Chung-Jong Kim, Hyesook Park, Eunhee Ha
The Ewha Medical Journal.2023;[Epub] CrossRef
 , Nackmoon Sung2
, Nackmoon Sung2 , Sun Hwa Lee3
, Sun Hwa Lee3 , Misun Chang4
, Misun Chang4 , Hee Jung Choi5
, Hee Jung Choi5 , Chung-Jong Kim5
, Chung-Jong Kim5 , Nam-Kyong Choi6
, Nam-Kyong Choi6 , Hanna Kim1
, Hanna Kim1 , Yi-Jun Kim1,7
, Yi-Jun Kim1,7 , Whanhee Lee8
, Whanhee Lee8 , Hyesook Park7,9,*
, Hyesook Park7,9,* , Eunhee Ha1,7,*
, Eunhee Ha1,7,*