Department of Surgery, Ewha Womans University School of Medicine, Seoul, Korea.
Copyright © 2017. Ewha Womans University School of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
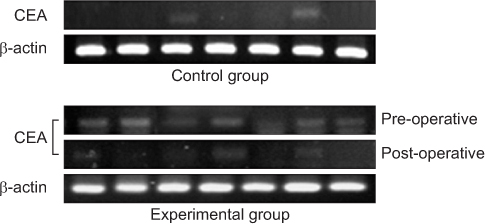
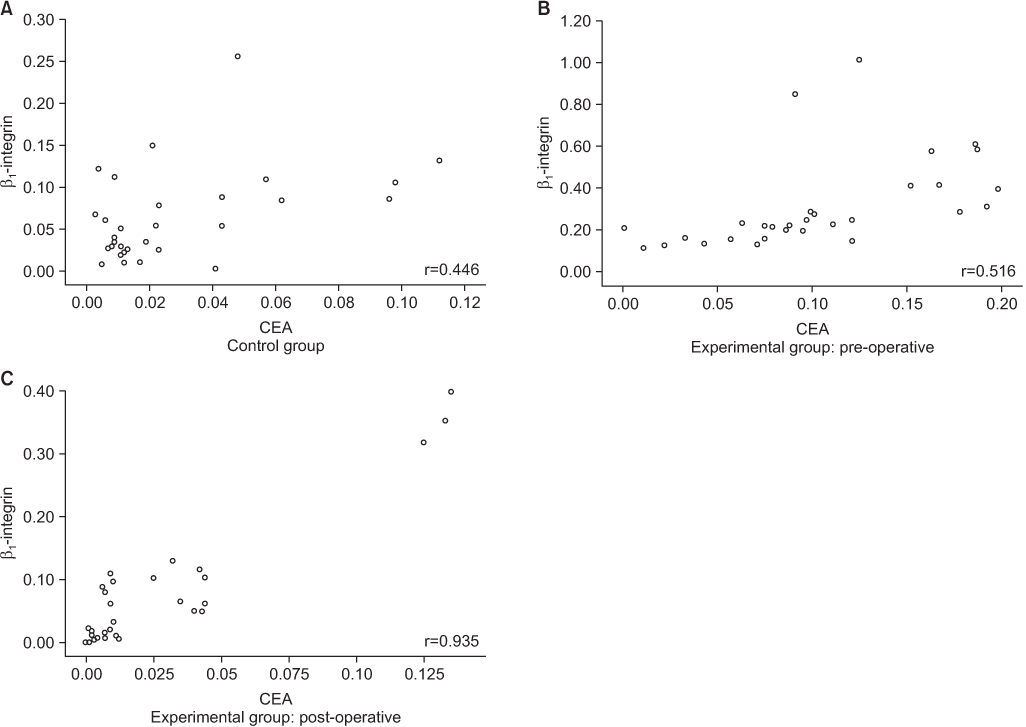
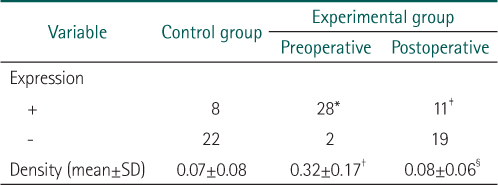
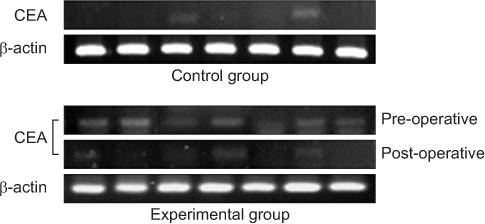
*Expression of carcinoembryonic antigen (CEA) was detected more in experimental group rather than in control group (P=0.008).
†Density of CEA was higher in experimental group as compared with the controls (P<0.001).
‡Expression of CEA was more significantly decreased after operation (P=0.032).
§Density of CEA was significantly decreased after operation (P<0.001).
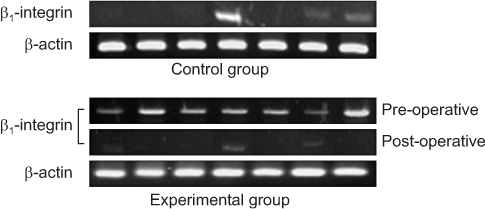
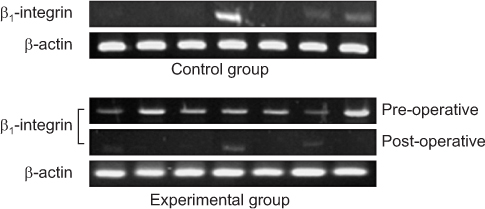
*Expression of β1-integrin was detected more in experimental group rather than in control group (P<0.001).
†Density of β1-integrin was higher in experimental group as compared with the controls (P<0.001).
‡Expression of β1-integrin was more significantly decreased after operation (P<0.001).
§Density of β1-integrin was significantly decreased after operation (P<0.001).

CEA, carcinoembryonic antigen; F, forward; R, reverse.
*Statistically not significant between experimental and control group.
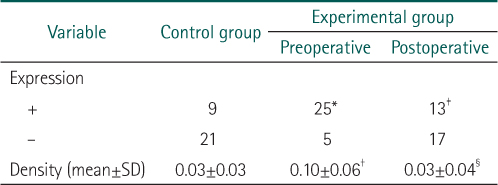
*Expression of carcinoembryonic antigen (CEA) was detected more in experimental group rather than in control group (P=0.008).
†Density of CEA was higher in experimental group as compared with the controls (P<0.001).
‡Expression of CEA was more significantly decreased after operation (P=0.032).
§Density of CEA was significantly decreased after operation (P<0.001).
*Expression of β1-integrin was detected more in experimental group rather than in control group (P<0.001).
†Density of β1-integrin was higher in experimental group as compared with the controls (P<0.001).
‡Expression of β1-integrin was more significantly decreased after operation (P<0.001).
§Density of β1-integrin was significantly decreased after operation (P<0.001).
CEA, carcinoembryonic antigen.
CEA, carcinoembryonic antigen; F, forward; R, reverse.
*Statistically not significant between experimental and control group.
*Expression of carcinoembryonic antigen (CEA) was detected more in experimental group rather than in control group (P=0.008). †Density of CEA was higher in experimental group as compared with the controls (P<0.001). ‡Expression of CEA was more significantly decreased after operation (P=0.032). §Density of CEA was significantly decreased after operation (P<0.001).
*Expression of β1-integrin was detected more in experimental group rather than in control group (P<0.001). †Density of β1-integrin was higher in experimental group as compared with the controls (P<0.001). ‡Expression of β1-integrin was more significantly decreased after operation (P<0.001). §Density of β1-integrin was significantly decreased after operation (P<0.001).
CEA, carcinoembryonic antigen.