 , An-Wen Chan2
, An-Wen Chan2 , Gary S. Collins3
, Gary S. Collins3 , Asbjørn Hróbjartsson4,5
, Asbjørn Hróbjartsson4,5 , David Moher6
, David Moher6 , Kenneth F. Schulz7
, Kenneth F. Schulz7 , Ruth Tunn1
, Ruth Tunn1 , Rakesh Aggarwal8
, Rakesh Aggarwal8 , Michael Berkwits9
, Michael Berkwits9 , Jesse A. Berlin10,11
, Jesse A. Berlin10,11 , Nita Bhandari12
, Nita Bhandari12 , Nancy J. Butcher13,14
, Nancy J. Butcher13,14 , Marion K. Campbell15
, Marion K. Campbell15 , Runcie C. W. Chidebe16,17
, Runcie C. W. Chidebe16,17 , Diana Elbourne18
, Diana Elbourne18 , Andrew Farmer19
, Andrew Farmer19 , Dean A. Fergusson20
, Dean A. Fergusson20 , Robert M. Golub21
, Robert M. Golub21 , Steven N. Goodman22, Tammy C. Hoffmann23
, Steven N. Goodman22, Tammy C. Hoffmann23 , John P. A. Ioannidis24
, John P. A. Ioannidis24 , Brennan C. Kahan25, Rachel L. Knowles26
, Brennan C. Kahan25, Rachel L. Knowles26 , Sarah E. Lamb27
, Sarah E. Lamb27 , Steff Lewis28, Elizabeth Loder29,30, Martin Offringa13
, Steff Lewis28, Elizabeth Loder29,30, Martin Offringa13 , Philippe Ravaud31, Dawn P. Richards32
, Philippe Ravaud31, Dawn P. Richards32 , Frank W. Rockhold33
, Frank W. Rockhold33 , David L. Schriger34, Nandi L. Siegried35
, David L. Schriger34, Nandi L. Siegried35 , Sophie Staniszewska36
, Sophie Staniszewska36 , Rod S. Taylor37
, Rod S. Taylor37 , Lehana Thabane38,39
, Lehana Thabane38,39 , David Torgerson40
, David Torgerson40 , Sunita Vohra41
, Sunita Vohra41 , Ian R. White25
, Ian R. White25 , Isabelle Boutron42
, Isabelle Boutron42
1Oxford Clinical Trials Research Unit, Centre for Statistics in Medicine, University of Oxford, Oxford, UK
2Department of Medicine, Women’s College Research Institute, University of Toronto, Toronto, ON, Canada
3United Kingdom EQUATOR Centre, Centre for Statistics in Medicine, University of Oxford, Oxford, UK
4Department of Clinical Research, Centre for Evidence-Based Medicine Odense and Cochrane Denmark, University of Southern Denmark, Odense, Denmark
5Open Patient data Explorative Network, Odense University Hospital, Odense, Denmark
6Centre for Journalology, Clinical Epidemiology Programme, Ottawa Hospital Research Institute, Ottawa, ON, Canada
7Department of Obstetrics and Gynecology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
8Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India
9Office of Science Dissemination, Centers for Disease Control and Prevention, Atlanta, GA, USA
10Department of Biostatistics and Epidemiology, School of Public Health, Center for Pharmacoepidemiology and Treatment Science, Rutgers University, New Brunswick, NJ, USA
11JAMA Network Open, Chicago, IL, USA
12Centre for Health Research and Development, Society for Applied Studies, New Delhi, India
13Child Health Evaluation Services, The Hospital for Sick Children Research Institute, Toronto, ON, Canada
14Department of Psychiatry, University of Toronto, Toronto, ON, Canada
15Aberdeen Centre for Evaluation, University of Aberdeen, Aberdeen, UK
16Project PINK BLUE-Health & Psychological Trust Centre, Abuja, Nigeria
17Department of Sociology and Gerontology, Miami University, Oxford, OH, USA
18Department of Medical Statistics, London School of Hygiene and Tropical Medicine, London, UK
19Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK
20Ottawa Hospital Research Institute, Ottawa, ON, Canada
21Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
22Department of Epidemiology and Population Health, Stanford University, Palo Alto, CA, USA
23Institute for Evidence-Based Healthcare, Faculty of Health Sciences and Medicine, Bond University, Robina, QLD, Australia
24Departments of Medicine, of Epidemiology and Population Health, of Biomedical Data Science, and of Statistics, and Meta-Research Innovation Center at Stanford (METRICS), Stanford University, Stanford, CA, USA
25MRC Clinical Trials Unit at University College London, London, UK
26University College London, UCL Great Ormond Street Institute of Child Health, London, UK
27NIHR Exeter Biomedical Research Centre, Faculty of Health and Life Sciences, University of Exeter, Exeter, UK
28Edinburgh Clinical Trials Unit, Usher Institute-University of Edinburgh, Edinburgh, UK
29The BMJ, BMA House, London, UK
30Harvard Medical School, Boston, MA, USA
31Université Paris Cité, Inserm, INRAE, Centre de Recherche Epidémiologie et Statistiques, Université Paris Cité, Paris, France
32Clinical Trials Ontario, MaRS Centre, Toronto, ON, Canada
33Duke Clinical Research Institute, Duke University Medical Center, Durham, NC, USA
34Department of Emergency Medicine, University of California, Los Angeles, CA, USA
35South African Medical Research Council, Cape Town, South Africa
36Warwick Applied Health, Warwick Medical School, University of Warwick, Coventry, UK
37MRC/CSO Social and Public Health Sciences Unit & Robertson Centre for Biostatistics, Institute of Health and Wellbeing, University of Glasgow, Glasgow, UK
38Department of Health Research Methods Evidence and Impact, McMaster University, Hamilton, ON, Canada
39St. Joseph’s Healthcare Hamilton, Hamilton, ON, Canada
40York Trials Unit, Department of Health Sciences, University of York, York, UK
41Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada
42Université Paris Cité and Université Sorbonne Paris Nord, Inserm, INRAE, Centre for Research in Epidemiology and Statistics (CRESS), Paris, France
43Centre d’Epidémiologie Clinique, Hôpital Hôtel Dieu, AP-HP, Paris, France

It is a Korean translation of the Hopewell S. et al. CONSORT 2025 statement: updated guideline for reporting randomized trials. Nat Med 2025 Jun;31(6):1776-1783.
© 2025 Ewha Womans University College of Medicine and Ewha Medical Research Institute
This is an open-access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Ethical approval
Ethical approval was granted by the Central University Research Ethics Committee, University of Oxford (R76421/RE001). All Delphi participants provided informed consent to participate.
Authors’ contribution
Conceptualization: SH, AWC, GSC, AH, DM, KFS, RT, RA, MB, NB, NJB, MKC, RCWC, DE, DAF, RMG, SNG, TCH, JPAI, BCK, RLK, SEL, SL, EL, MO, PR, DPR, FWR, DLS, NLS, SS, RST, LT, DT, SV, IRW, IB. Funding acquisition: SH, GSC. Investigation: SH, AWC, GSC, AH, DM, KFS, RT, RA, MB, NB, NJB, MKC, RCWC, DE, DAF, RMG, SNG, TCH, JPAI, BCK, RLK, SEL SL, EL, MO, PR, DPR, FWR, DLS, NLS, SS, RST, LT, DT, SV, IRW, IB. Methodology: SH, AWC, GSC, AH, DM, KFS, RT, RA, MB, NB, NJB, MKC, RCWC, DE, DAF, RMG, SNG, TCH, JPAI, BCK, RLK, SEL, SL, EL, MO, PR, DPR, FWR, DLS, NLS, SS, RST, LT, DT, SV, IRW, IB. Project administration: SH, RT. Supervision: SH, AWC, GSC, DM, KFS, IB. Writing–original draft: SH, AWC, GSC, AH, DM, KFS, RT, IB. Writing–review & editing: SH, AWC, GSC, AH, DM, KFS, RT, RA, MB, JAB, NB, NJB, MKC, RCWC, DE, AF, DAF, RMG, SNG, TCH, JPAI, BCK, RLK, SEL, SL, EL, MO, PR, DPR, FWR, DLS, NLS, SS, RST, LT, DT, SV, IRW, IB.
Conflict of interest
I have read the journal’s policy and the authors of this manuscript have the following competing interests: support from MRC-NIHR for the submitted work. SH, IB, AWC, AH, KFS, and DM are members of the SPIRIT-CONSORT executive group. SH, IB, AWC, AH, KFS, GSC, DM, MKC, NJB, MO, RST, and SV are involved in the development, update, implementation, and dissemination of several reporting guidelines. GSC is the director of the UK EQUATOR Centre, a statistical editor for The BMJ and NIHR Senior Investigator, DM is the director of the Canadian EQUATOR Centre, and member of The BMJ’s regional advisory board for North America, IB is deputy director and PR is director of the French EQUATOR Centre, TCH is director of the Australasian EQUATOR Centre, JPAI is director of the US EQUATOR Centre. RA is president of the World Association of Medical Editors. MKC is chair of the MRC-NIHR: Better Methods Better Research funding panel. RCWC is executive director of Project PINK-BLUE, which receives funding from Roche-Product. AF is director of the UK National Institute for Health and Care Research Health Technology Assessment Programme. DPR is a full time employee of Five02 Laboratories, which under contract to Clinical Trials Ontario provides services related to patient and public engagement; and is the volunteer vice president of the Canadian Arthritis Patient Alliance, which receives funding through independent grants from pharmaceutical companies. IRW was supported by the MRC Programmes MCUU00004/07 and MCUU00004/09. DLS is JAMA associate editor and receives editing stipends from JAMA and Annals of Emergency Medicine.
Funding
The 2025 update of SPIRIT and CONSORT was funded by the MRC-NIHR: Better Methods, Better Research (MR/W020483/1). The funder reviewed the design of the study but had no role in the collection, analysis, or interpretation of the data; in the writing of the report; or in the decision to submit the article for publication. The views expressed are those of the author(s) and not necessarily those of the NIHR, the MRC, or the Department of Health and Social Care.
Data availability
Not applicable.
Acknowledgments
SPIRIT-CONSORT executive group: Sally Hopewell, University of Oxford, UK; An-Wen Chan, University of Toronto, Canada; Asbjørn Hróbjartsson, University of Southern Denmark, Odense, Denmark; David Moher, University of Ottawa, Canada; Kenneth Schulz, University of North Carolina at Chapel Hill, USA; Isabelle Boutron, Université Paris Cité, France. Gary Collins and Ruth Tunn, both of University of Oxford, UK, were also involved in leading the SPIRIT and CONSORT 2025 update. SPIRIT-CONSORT 2025 consensus meeting participants: Rakesh Aggarwal, Jawaharlal Institute of Postgraduate Medical Education and Research, India; Michael Berkwits, Office of the Centers for Disease Control and Prevention, USA (formally JAMA and the JAMA Network at time of consensus meeting); Jesse A Berlin, Rutgers University/JAMA Network Open USA; Nita Bhandari, Society for Applied Studies, India; Nancy J Butcher, The Hospital for Sick Children, Canada; Marion K Campbell, University of Aberdeen, UK; Runcie W Chidebe, Project PINK BLUE, Nigeria/Miami University, Ohio, USA; Diana Elbourne, London School of Hygiene and Tropical Medicine, UK; Andrew J Farmer, University of Oxford, UK; Dean A Fergusson, Ottawa Hospital Research Institute, Canada; Robert M Golub, Northwestern University, USA; Steven N Goodman, Stanford University, USA; Tammy C Hoffmann, Bond University, Australia; John PA Ioannidis, Stanford University, USA; Brennan C Kahan, University College London, UK; Rachel L Knowles, University College London, UK; Sarah E Lamb, University of Exeter, UK; Steff Lewis, University of Edinburgh, UK; Elizabeth Loder, The BMJ, UK; Martin Offringa, Hospital for Sick Children Research Institute, Canada; Dawn P Richards, Clinical Trials Ontario, Canada; Frank W Rockhold, Duke University, USA; David L Schriger, University of California, USA; Nandi L Siegfried, South African Medical Research Council, South Africa; Sophie Staniszewska, University of Warwick, UK; Rod S Taylor, University of Glasgow, UK; Lehana Thabane, McMaster University/St. Joseph’s Healthcare, Canada; David Torgerson, University of York, UK; Sunita Vohra, University of Alberta, Canada; and Ian R White, University College London, UK. We dedicate CONSORT 2025 to the late Doug Altman who was instrumental in the development of the SPIRIT and CONSORT statements and whose files and correspondence contributed to this update following his death. We gratefully acknowledge the contributions of all those who participated in the SPIRIT-CONSORT 2025 update Delphi survey. We also acknowledge Camilla Hansen Nejstgaard for conducting the scoping review of the literature to identify suggested changes to CONSORT 2010 and for comparing CONSORT 2025 with RoB2, Lasse Østengaard for developing the SCEB (SPIRIT-CONSORT Evidence Bibliographic) database of empirical evidence to support the development of CONSORT, Jen de Bayer and Patricia Logullo for their involvement during the consensus meeting, and Lina El Ghosn for initial drafting of the expanded CONSORT checklist.
Patient and public involvement: The CONSORT 2025 checklist items and the explanations here were developed using input from an international Delphi survey and consensus meeting. The Delphi survey was advertised via established patient and public involvement (PPI) networks, and 17 respondents self-identified as a “patient or public representative” and completed the Delphi survey. In addition, three of the participants in the expert consensus meeting were patient or public representatives who were leaders in advancing PPI. Dissemination to participants and related patient and public communities: CONSORT 2025 will be disseminated via a new website, consort-spirit.org, which will include materials designed for patients and the public.


| 섹션/주제 | No. | CONSORT 2025 체크리스트 항목 설명 |
|---|---|---|
| 제목 및 초록 | ||
| 제목 및 구조화된 초록 | 1a | 무작위 임상시험으로서의 식별 |
| 1b | 임상시험 설계, 방법, 결과 및 결론의 구조화된 요약 | |
| 오픈 사이언스 | ||
| 임상시험 등록 | 2 | 임상시험 등록부 이름, 식별번호(URL 포함) 및 등록날짜 |
| 프로토콜 및 통계 분석계획 | 3 | 시험 프로토콜 및 통계 분석계획에 접근할 수 있는 위치 |
| 데이터 공유 | 4 | 비식별화된 개별 참가자 데이터(데이터 사전 포함), 통계 코드 및 기타 자료에 접근할 수 있는 위치 및 방법 |
| 자금 및 이해관계 | 5a | 자금 및 기타 지원 출처(예: 의약품 공급) 및 시험의 설계, 수행, 분석 및 보고에서 자금 제공자의 역할 |
| 5b | 원고 저자의 재정 및 기타 이해관계 | |
| 서론 | ||
| 배경 및 근거 | 6 | 과학적 배경 및 근거 |
| 목적 | 7 | 이익 및 위해와 관련된 구체적인 목표 |
| 방법 | ||
| 환자 및 대중 참여 | 8 | 시험의 설계, 수행 및 보고에 대한 환자 또는 대중 참여 세부 사항 |
| 시험 설계 | 9 | 시험 유형을 포함한 시험 설계 설명(예: 평행군, 교차), 할당 비율 및 프레임워크(예: 우월성, 동등성, 비열등성, 탐색적) |
| 시험 프로토콜 변경 | 10 | 시험 시작 후 미리 지정되지 않은 결과 또는 분석을 포함한, 시험에 대한 중요한 변경사항 및 이유 |
| 시험 설정 | 11 | 설정(예: 지역사회, 병원) 및 위치(예: 국가, 시험기관) |
| 자격 기준 | 12a | 참가자 자격 기준 |
| 12b | 해당되는 경우, 기관 및 중재를 제공하는 개인(예: 외과의, 물리치료사)에 대한 자격 기준 | |
| 중재 및 비교군 | 13 | 복제를 허용하기에 충분한 세부 정보가 포함된 중재 및 비교군. 해당되는 경우, 중재 및 비교자를 설명하는 추가 자료(예: 중재 매뉴얼)에 접근할 수 있는 곳 |
| 결과 | 14 | 특정 측정 변수(예: 수축기 혈압), 분석 지표(예: 기준치, 최종값, 이벤트까지의 시간 변화), 집계방법(예: 중앙값, 비율) 및 각 결과에 대한 시점 |
| 위해 | 15 | 위해를 정의하고 평가한 방법(예: 체계적, 비체계적) |
| 표본 크기 | 16a | 표본 크기 계산을 뒷받침하는 모든 가정을 포함하여 표본 크기를 결정한 방법 |
| 16b | 중간 분석 및 중단 지침에 대한 설명 | |
| 무작위 배정: | ||
| 시퀀스 생성 | 17a | 무작위 할당 시퀀스를 생성한 사람 및 사용된 방법 |
| 17b | 무작위 할당 유형 및 제한 사항(예: 층화, 차단 및 블록 크기) | |
| 할당 은폐 메커니즘 | 18 | 무작위 할당 시퀀스를 구현하는 데 사용된 메커니즘(예: 중앙 컴퓨터/전화, 순차적으로 번호가 매겨진 불투명하고 밀폐된 용기), 중재가 배정될 때까지 순서를 은폐하는 모든 단계를 설명 |
| 구현 | 19 | 등록한 직원과 참여자를 중재에 배정하는 직원이 무작위 배정 순서에 접근할 수 있는지 여부 |
| 눈가림 | 20a | 중재 배정 후의 눈가림 대상(예: 참가자, 의료 제공자, 결과 평가자, 데이터 분석가) |
| 20b | 눈가림이 이루어진 경우, 눈가림이 어떻게 시행되었는지와 중재 간 유사성에 대한 설명 | |
| 통계방법 | 21a | 위해를 포함한 일차 및 이차 결과에 대한 그룹을 비교하는 데 사용한 통계적 방법 |
| 21b | 각 분석에 포함되는 사람(예: 모든 무작위 참가자) 및 그룹에 대한 정의 | |
| 21c | 분석에서 누락된 데이터를 처리한 방법 | |
| 21d | 추가 분석을 위한 방법(예: 하위 그룹 및 민감도 분석), 사전 지정과 사후 분석을 구분 | |
| 결과 | ||
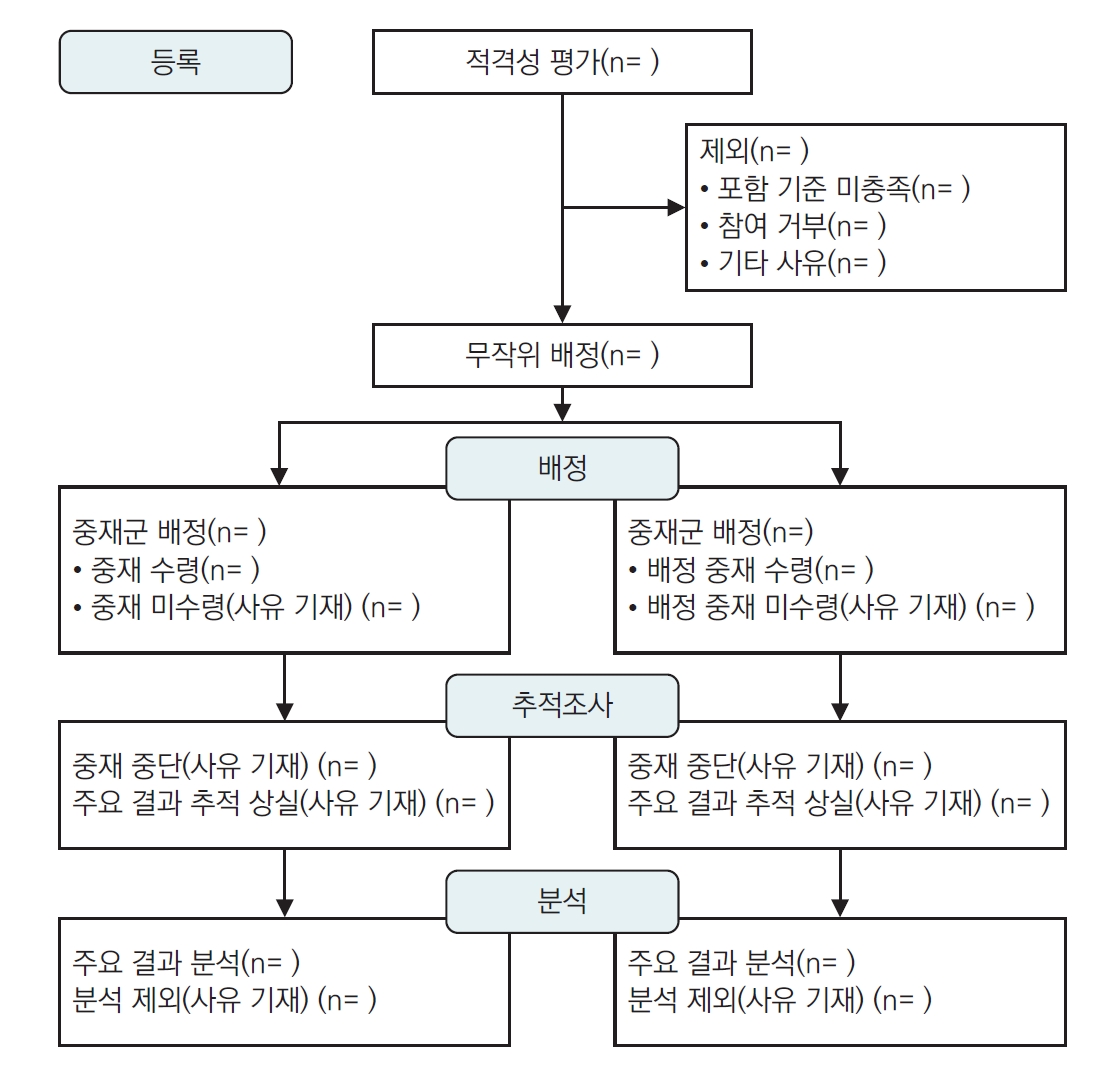
| 흐름도를 포함한 참가자 흐름 | 22a | 각 그룹에 대해 무작위로 배정되고, 의도된 중재를 받고, 일차 결과 분석에 포함된 참가자 수 |
| 22b | 각 그룹에 대해 무작위 배정 후의 손실 및 제외와 그 이유 | |
| 모집 | 23a | 혜택 및 피해 결과에 대한 모집 및 추적 기간을 정의하는 날짜 |
| 23b | 관련이 있는 경우 시험이 종료되거나 중단된 이유 | |
| 중재 및 비교 대상 제공 | 24a | 실제로 시행된 중재 및 비교 대상(예: 적절한 경우, 누가 중재/비교약을 제공했는지, 참가자가 어떻게 준수했는지, 의도대로 제공되었는지 여부[충실도]) |
| 24b | 각 그룹이 시험기간에 받은 병용 치료 | |
| 기준 데이터 | 25 | 각 그룹의 기준 인구통계 및 임상 특성을 보여주는 표 |
| 분석된 숫자, 결과 및 추정 | 26 | 각 1차 및 2차 결과, 그룹별로: |
| - 분석에 포함된 참가자 수 | ||
| - 결과 시점에 사용 가능한 데이터를 가진 참가자 수 | ||
| - 각 그룹에 대한 결과 및 추정 효과 크기와 그 사전값 | ||
| 위해 | 27 | 각 그룹에서 발생한 모든 위해 또는 의도치 않은 사건 |
| 보조 분석 | 28 | 사전 지정 분석과 사후 분석을 구별하여 수행된 기타 분석(하위 그룹 및 민감도 분석 포함) |
| 고찰 | ||
| 해석 | 29 | 결과에 부합하고, 이점과 위해의 균형을 맞추며, 기타 관련 근거를 고려한 해석 |
| 한계 | 30 | 잠재적 편향, 부정확성, 일반화 가능성 및 관련 있는 경우, 분석의 다중성 원인을 다루는 시험 한계 |